Laminar Air Flow is a critical technology used in scientific, medical, and industrial environments to create a controlled and contamination-free airflow. This specialized system ensures a consistent and unidirectional flow of air, minimizing turbulence and preventing the entry and spread of particles and contaminants. Equipped with high-efficiency filters, Laminar Air Flow systems effectively remove dust, microbes, and allergens, maintaining a clean and sterile working environment. They are widely employed in cleanrooms, laboratories, hospitals, pharmaceutical manufacturing facilities, and electronics assembly lines, where maintaining stringent air quality standards is essential. By providing precise airflow control and advanced filtration capabilities, Laminar Air Flow technology safeguards sensitive processes, prevents product contamination, and promotes the health and safety of personnel. Its crucial role in maintaining the purity and integrity of critical operations has made it an indispensable asset across a range of industries. Trustworthy and efficient, Laminar Air Flow is the solution for ensuring optimal air quality and maintaining the highest standards of cleanliness.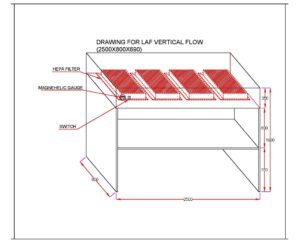
Laminar Air Flow Principle
The principle of Laminar Air Flow is based on the creation of a controlled and uniform airflow pattern within a defined space. The system operates on the concept of laminar flow, which refers to the movement of air in parallel layers with minimal mixing or turbulence.
Laminar Air Flow systems typically consist of a specialized airflow unit equipped with high-efficiency filters. The air is drawn into the unit and passes through these filters, which remove contaminants such as dust, microbes, and particles.
Once the air is filtered, it is directed through a series of adjustable vents or perforations in a manner that creates a consistent and unidirectional flow. This means that the air moves in a straight path without any significant deviations or swirls.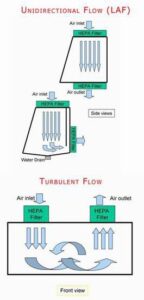
To achieve laminar flow, the system maintains a specific air pressure differential between the clean area and the surrounding environment. This pressure differential ensures that clean air flows from the filtered area into the controlled space, preventing the ingress of contaminants.
By controlling the direction and velocity of the airflow, laminar flow systems establish a clean and sterile environment, free from particles that could compromise sensitive processes or contaminate products. This principle is crucial in applications where maintaining strict air quality standards is vital, such as cleanrooms, laboratories, and healthcare facilities.
Overall, the principle of Laminar Air Flow revolves around precise airflow control, advanced filtration, and the creation of a unidirectional and contamination-free environment to ensure optimal air quality and protect critical operations.
Types of Laminar Air Flow
There are several types of Laminar Air Flow chambers or systems commonly used in various industries. The specific type of chamber selected depends on the intended application, space constraints, and other factors. Here are some commonly encountered types:
- Horizontal Laminar Flow Chambers: In this configuration, the air flows horizontally from the back wall of the chamber towards the front opening. It provides a clean and sterile work area for activities such as sample preparation, electronics assembly, and other tasks where product protection is essential.
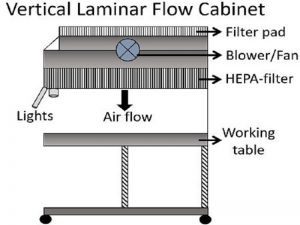
- Vertical Laminar Flow Chambers: These chambers feature a vertical airflow pattern, where the air descends from the top of the chamber towards the work surface. Vertical flow chambers are often utilized in microbiology labs, pharmacies, and medical facilities for activities like sterile media preparation, drug compounding, and tissue culture work.
- Biological Safety Cabinets (BSC): BSCs provide both Laminar Air Flow and containment features. They are equipped with HEPA filters to maintain a sterile environment, and they also incorporate airflow systems that protect the operator and the surrounding environment from potentially hazardous biological materials. BSCs are commonly found in medical, pharmaceutical, and research laboratories.
- Clean Benches: Clean benches are compact Laminar Air Flow chambers typically used for smaller-scale applications. They provide a localized clean and sterile environment for tasks such as sample handling, testing, or inspection. Clean benches can be either horizontal or vertical flow configurations.
- Mini Environments: Mini environments are small, enclosed Laminar Air Flow systems designed to provide localized clean zones within a larger workspace. They are often used in electronics manufacturing or assembly processes, where specific areas require strict air quality control.
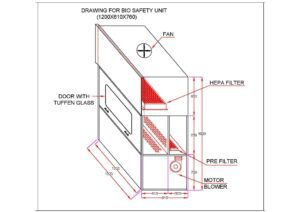
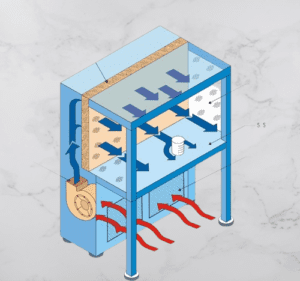
Major Components of the Laminar Air Flow Chamber
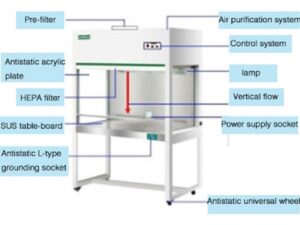
A Laminar Air Flow system comprises several essential components that work together to create and maintain a controlled and contamination-free airflow. The key components include:
- Air Filtration System: The filtration system is a crucial component of Laminar Air Flow. It typically includes high-efficiency particulate air (HEPA) filters. These filters effectively capture and remove airborne particles, such as dust, microbes, allergens, and other contaminants, ensuring clean and sterile airflow.
- The Blower or Fan Unit: The blower or fan unit is responsible for drawing in the ambient air and pushing it through the filtration system. It generates the airflow required for the Laminar Air Flow system and ensures a consistent and controlled velocity of air movement.
- Airflow Direction Control: Laminar Air Flow systems employ various methods to control the direction of airflow. This can include adjustable vents, perforated plates, or specific airflow patterns created by the design of the system. The goal is to establish a unidirectional flow that moves in a straight path without turbulence or mixing.
- Work Surface or Clean Area: The work surface or clean area is the designated space where activities requiring a controlled environment take place. It can be a bench, table, or enclosed chamber, depending on the specific application. The work surface is designed to be easy to clean and maintain sterility.
- Pressure Differential Control: Laminar Air Flow systems often rely on pressure differentials to maintain the desired airflow direction and prevent the ingress of contaminants. This can involve maintaining a higher air pressure in the clean area compared to the surrounding environment or adjacent areas.
- Control Panel: The control panel houses the controls and settings for the Laminar Air Flow system. It allows the user to adjust parameters such as airflow velocity, pressure differentials, and other system-specific settings to ensure optimal performance and adherence to required standards.
Validation Steps for the Qualification of LAF:
| Validation Step | Description |
|---|---|
| Pre-Installation Checks | Ensure that the Laminar Air Flow system meets specifications and requirements before installation. |
| Installation Qualification (IQ) | Verify that the system is installed correctly, including proper positioning, electrical connections, and filter installation. |
| Operational Qualification (OQ) | Test the system under normal operating conditions to ensure it performs as intended. This includes airflow velocity, uniformity, and filter integrity tests. |
| Performance Qualification (PQ) | Validate the system’s performance over an extended period, typically by monitoring and documenting parameters such as airflow velocity, particle counts, and microbial levels. |
| HEPA Filter Integrity Test | Conduct a challenge test to confirm the integrity of HEPA filters and their ability to remove particles of specified sizes. |
| Airflow Pattern Test | Verify the unidirectional airflow pattern by using smoke or other visual indicators to ensure proper airflow direction and absence of turbulence. |
| Particulate Monitoring | Measure and monitor the level of airborne particles within the clean area using particle counters to ensure compliance with specified limits. |
| Microbial Monitoring | Perform regular microbial sampling to assess the microbial contamination levels within the clean area and ensure compliance with specified standards. |
| Alarm and Interlock Testing | Test the functionality of alarms, interlocks, and safety features to ensure prompt detection and response to any abnormal conditions. |
| Documentation | Maintain comprehensive records of all validation activities, including protocols, test results, deviations, and corrective actions. |
Laminar air flow uses / laminar airflow use :
| Application | Description |
|---|---|
| Aseptic Processing | LAF systems are crucial in aseptic processing areas, such as sterile manufacturing, filling lines, and compounding of parenteral products, to maintain a sterile environment during critical processes. |
| Cleanroom Operations | LAF systems are extensively used in cleanrooms to control airborne contamination and maintain the required air quality for activities like formulation, packaging, and quality control. |
| Microbiological Testing | LAF systems provide a sterile environment for performing microbiological testing, including microbial culture, media preparation, and plate pouring, to minimize the risk of contamination and accurate results. |
| Tissue Culture | LAF systems are utilized in tissue culture laboratories to create a controlled environment for cell culture, cell manipulation, and propagation of cell lines, ensuring sterile conditions and preventing cross-contamination. |
| Sterile Compounding | LAF systems play a critical role in pharmacies and compounding facilities where sterile compounding of medications, including intravenous preparations and customized dosage forms, takes place. |
| Research and Development | LAF systems are used in pharmaceutical research and development labs to maintain a controlled and particle-free environment for sensitive experiments, sample preparation, and formulation development. |
| Quality Control and Analysis | LAF systems are employed in quality control laboratories to prevent contamination during analytical testing, such as dissolution testing, stability studies, and particle size analysis. |
| Vaccine Production | LAF systems are utilized in vaccine production facilities to ensure a sterile environment during the manufacturing, filling, and packaging processes to maintain the safety and efficacy of vaccines. |
| Gene Therapy and Cell-based Therapies | LAF systems are essential for maintaining aseptic conditions during the production, manipulation, and handling of genetically modified organisms and cell-based therapies. |
| Ophthalmic Solution Preparation | LAF systems are used in facilities where ophthalmic solutions, such as eye drops and ointments, are prepared to maintain sterility and prevent contamination. |
Laminar air flow principle working and uses
Laminar Air Flow (LAF) is based on the principle of creating a controlled and uniform airflow pattern in a defined space. It involves directing air in a consistent, unidirectional flow to minimize turbulence and prevent the entry and spread of contaminants. LAF systems utilize high-efficiency filters to remove airborne particles, such as dust, microbes, and allergens, ensuring a clean and sterile working environment.
Laminar air flow definition
The working of LAF involves drawing in ambient air, filtering it through HEPA filters, and then directing it through adjustable vents or perforations to create a laminar flow pattern. This laminar flow moves in a straight path without significant deviations, ensuring a clean zone where particles are minimized.
LAF systems find extensive use in various industries, including pharmaceuticals, cleanrooms, laboratories, hospitals, and electronics manufacturing. They are employed in applications such as aseptic processing, cleanroom operations, sterile compounding, microbiological testing, and quality control. LAF systems help maintain strict air quality standards, protect sensitive processes, prevent product contamination, and ensure the well-being of personnel working in controlled environments.
Overall, the principle of Laminar Air Flow revolves around precise airflow control, advanced filtration, and the creation of a unidirectional and contamination-free environment, making it an essential tool in industries that require stringent air quality control.
Frequently Asked Questions:
What is the purpose of a Laminar Air Flow system in pharmaceuticals?
Answer: The purpose of a Laminar Air Flow system in pharmaceuticals is to create a controlled and sterile environment, preventing the entry of contaminants and maintaining air quality for critical processes.
What is the role of HEPA filters in a Laminar Air Flow system?
Answer: HEPA filters in a Laminar Air Flow system remove airborne particles, including dust, microbes, and allergens, ensuring a clean and sterile airflow.
How does a Laminar Air Flow system achieve unidirectional airflow?
Answer: Laminar Air Flow systems achieve unidirectional airflow by using adjustable vents or perforations to direct the airflow in a straight path, minimizing turbulence and mixing.
What is the recommended air velocity in a Laminar Air Flow system for pharmaceutical applications?
Answer: The recommended air velocity in a Laminar Air Flow system for pharmaceutical applications is typically between 0.36-0.54 m/s (70-100 ft/min) to ensure efficient particle control.
How often should the HEPA filters be replaced in a Laminar Air Flow system?
Answer: The frequency of HEPA filter replacement depends on factors such as usage, environmental conditions, and regulatory requirements. Typically, filters are replaced every 6 to 12 months or as specified by the manufacturer.
What tests are performed to validate the performance of a Laminar Air Flow system?
Answer: Performance validation tests for a Laminar Air Flow system include airflow velocity measurements, air particle counts, HEPA filter integrity testing, and smoke studies to verify the unidirectional airflow pattern.
What are the considerations for positioning a Laminar Air Flow system in a pharmaceutical facility?
Answer: Laminar Air Flow systems should be positioned away from doors, high-traffic areas, and potential sources of contamination. They should also be installed to minimize airflow disruptions caused by room conditions or equipment.
Can Laminar Air Flow systems eliminate all airborne contaminants in a pharmaceutical setting?
Answer: Laminar Air Flow systems can significantly reduce airborne contaminants, but they cannot eliminate all contaminants. Proper gowning, cleaning, and adherence to standard operating procedures are also necessary for contamination control.
What is the purpose of conducting airflow pattern tests in a Laminar Air Flow system?
Answer: Airflow pattern tests in a Laminar Air Flow system confirm the unidirectional airflow pattern and ensure the absence of turbulence or stagnant zones, which could lead to the spread of contaminants.
What are the maintenance requirements for Laminar Air Flow systems in pharmaceuticals?
Answer: Maintenance requirements include regular filter replacement, cleaning of vents or perforations, calibration of monitoring devices, and adherence to a documented maintenance schedule according to manufacturer guidelines and regulatory requirements.

Good post! We are linking to this great content on our site.
Keep up the good writing.
Hi to all, for the reason that I am genuinely keen of reading this weblog’s post to be updated on a regular
basis. It includes pleasant stuff.
Wow, this is a fantastic explanation of the Laminar Air Flow Principle! I never thought I’d understand it so easily, but Flair Pharma’s clear examples and diagrams really helped. Can’t wait to apply this in my own work!