Vendor Qualification & Management SOP
- OBJECTIVE
- To lay down a procedure for identifying, selecting, evaluating, and qualifying vendors and contract services provider/ laboratory.
- SCOPE
- This SOP shall be applicable to qualify vendors of all the raw, primary, printed packaging material and miscellaneous materials procured. The SOP is also applicable to qualify the Laboratory contracts, and all API, Excipients & vendors.
- RESPONSIBILITY
- Head-Purchase/designee shall be responsible for the identification and selection of vendors.Head-QA/designee shall be responsible for the evaluation, qualification, and approval of the Vendor.
- Head-Production /Head Packaging /Designee shall perform the trial batch study of the sample supplied by the vendor.
- Purchase department shall be responsible for the initiation of the vendor qualification process in coordination with QA and other departments.
- QC shall be responsible for the analysis of the samples.
- ACCOUNTABILITY
- Head QA/Designee shall be accountable for this SOP.
- DEFINITIONS
- Vendor:- Vendor is a manufacturer, who produces the intended material, Vendor may supply material directly or through a trader in its original packaging.
- Active Pharmaceutical Ingredient (API): Active Pharmaceutical Ingredients (API) is the ingredients in a Pharmacopoeial drug that is biological active or produce its effects.
- Excipients : An inactive substances that serves as the vehicle or medium for a drug or other active substances.
- PROCEDURE
- All the Vendors of raw material, primary and printed packaging material shall go through the vendor qualification procedure.
- Vendor qualification process is necessary for the following –
- New material from a new vendor / existing vendor.
- Existing material from a new vendor (alternate vendor).
- Change in manufacturing site of vendor.
- Routine Re-qualification.
- The vendors shall be identified by the purchase department within the country or abroad from where the material shall be procured.
- The full procedure of vendor approval for bulk consistent supply consists of four stages: vendor selection, trial of their sample and its study, vendor visit and final stage of approval/ non approval.
The information flow shall be as follow:
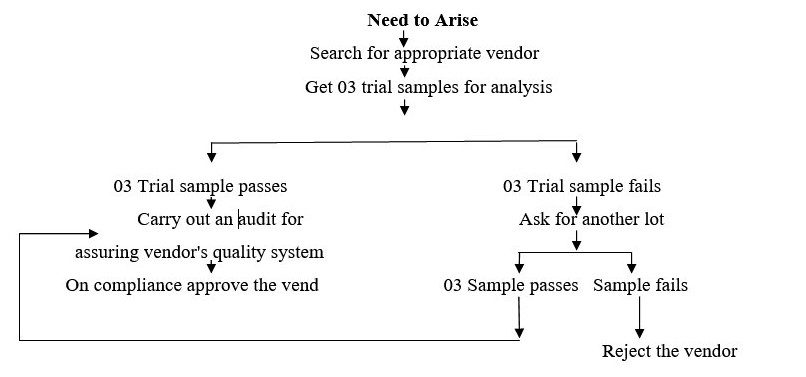
- On arising a need for finding a new vendor by purchase department along with the existing one to meet our larger requirements, or the need to look for a new vendor to fulfill new material requirements or to have the vendor approved as a second option of supplying goods, the purchase officer shall search for the companies/ agencies manufacturing or supplying these. This shall be done through searching the internet or the catalogues for range of products.
Coordination for the 03 trial sample quantity for that particular material shall be done with the QC Head.
- The Purchase officer shall correspond with the vendor and introduce our company and specify requirements, also requesting to send a 03 trial sample of the qty. agreed with QC Head along with the material specification/ COA and test method with reference (If any).
- The QC Head shall arrange for the analysis of the same as per our in house specifications and on compliance as per our defined standards regarding the quality and the practical suitability of that material, he shall intimate the Purchase department about the status of the same. The outcome / result shall be discussed with the Head the Purchase department about the status of the same. The outcome / result shall be discussed with the Head Production.
- The chemical and physical parameters of such samples shall be evaluated by QC department and the data shall be compared with COA supplied by the vendor.If quality of such material is found satisfactory, the same shall be notifying to QA department.
- Purchase department shall send the Vendor Questionnaire for raw material as per Annexure- III, Vendor Questionnaire for packing and printing material as per Annexure- IV, Quality Agreement as per Annexure- VII and Questionnaire for screening of TSE BSE as per Annexure- X to the vendor before the visit/ audit and get it filled satisfactory.
- Vendor shall fill the questionnaire, quality agreement, Questionnaire for screening of TSE BSE and send back the filled annexure to the purchase department or QA department through mail or original copy by courier along with the document required for vendor approval.
- After receiving the questionnaires, purchase department shall forward it to QA department for Review and evaluation.Site QA shall review the Vendor Assessment Questionnaire and other required documents i.e. TSE/ BSE certification and other certification & documents etc. (as applicable).After complying results of the analysis the QC Head shall intimate the Purchase officer/ Head about the same. And a visit shall be planned to the manufacturing site with the supplier, with prior permission of Head Production Simultaneously one lot of material shall be ordered.
- The planning of that visit which is to be conducted is then discussed with the Quality Assurance Head. The QA Officer, who has already been trained in conducting the audits, shall be called and the purpose of the audit shall be discussed with him by QA Head. QA officer shall provide him / her check list for the audit and tell him / what all is to be done. (This visit / audit may be conducted at any time after 03 trial sample approval to any time after consistent few supply receipt at the plant. It can also be repeated if required in case of any non compliance to the product lot supplied or on receipt of market complaint related to the product we purchase from them).
- On approval of the vendor after the visit/ audit, the vendor’s name shall be added to the list of approved vendors (Approved Vendor List Raw Materials as per Annexure-I & Approved Vendor List for Packing Materials as per Annexure-II ). And the Controlled copy of this list of Approved vendors shall be sent to the Warehouse Head as well as Purchase Head. We may or may not choose to procure goods from the listed supplier because of some reason like cost difference, delivery lead-time etc.
- Take risk analysis report from API manufacturer and accept the report as applicable for that API in Indus.
- Manufacturer provide 2 or 3 risk analysis reports for audit done.
- Note: Vendor shall be approved on the basis of vendor questionnaire (desk audit), analytical testing results or on basis of regulatory approval, without performing of site audit and vendor audit shall be planned as per schedule Annexure- VIII.
- The further deletion from the vendor’s list could be result of any non conformance observed during the visit &/or consistently 3 lot rejects of the same supplier of the same material or any other commercial reason (after consultation with the Production Head in case of commercial reason) In case of any quality issue the QC Head shall have the full authority to intimate the Purchase Head to further not to purchase any lot.
- The Purchase, Quality Control/ Quality Assurance and Production team (if required) shall do the evaluation of the vendor jointly.
Selection criteria for Auditors/Auditor team:
- Head-QA/ Designee shall identify personnel for vendor audits based on qualification, communication or presentation skills, experience, knowledge and expertise or combination of all in the different areas of operation from various functions like Quality Assurance, Quality Control and manufacturing.
- Identified personnel shall be qualified as per Format as per Annexure-XI.
Note: Person should have at least 05 years of overall experience in the process and operations or in relevant area.
- Auditors shall have relevant experience in Pharmaceutical organizations, auditing skills, knowledge and training on relevant regulatory, cGMP requirements, ICHQ7, ICHQ10, EU GMP and 21 CFR part 11 requirements.
- The person, who does not have relevant experience, shall be trained on guidelines/standards and practical training and shall be experienced as auditor upon completion of trainings, the person shall be certified as qualified auditor by assessing theoretical and practical knowledge. Certification of auditor shall be documented as per as per Annexure-XI.
- Auditor list shall be prepared as per Annexure- XII.
- Head-QA/ Designee shall be responsible for selecting the auditing team.
- Vendor Audit shall be conducted based on following, but not limited to :
- Adequacy of building and Facilities.
- Location and surrounding
- Ventilation System
- Air filtration system
- Air heating and cooling system
- Lighting & Sanitation Water Supply
- Disposal of wastageToilet and bathroom facility
- Washing facilities and space
- Segregation of various section
- House keeping
- General cleanliness
- ClothingCleaning
- Ingredients/disinfectant used
- Cleanliness of Machine
- Written cleaning process
- Adequate facility for First Aid
- General cleanliness
- Personnel
- Adequacy of qualified/trained/experienced personnel
- Personnel Hygiene
- Personnel behavior
- Equipments
- Equipments are of appropriate design and adequate size.
- Proper location of equipment to facilitate operation, cleaning and maintenance.
- Whether or not the equipment construction material is likely to affect the quality parameters of material.
- Follow up of appropriate cleaning and maintenance schedule to prevent malfunctioning and contamination of product.
- Material/ Components
- Proper identification system of material to avoid errors at in issue?Calibration of balance with standard weight’s & measurements authorized by Government)
- Adequate segregation of material to avoid mix up.
- Availability of dispensing area
- Follow up of approved written sampling procedure.
- Adequate identification for materials whether they are approved or rejected?
- Proper segregation of released items from material under test and disposal?
- Adequate Control of rejected material.
- Follow up of first-in first-out system.
- Proper handling & storage of materials to prevent damage, contamination or loss?
- Adequate retesting frequencies for approved materials.
- Manufacturing
- Adequacy and maintenance of manufacturing processes to produce quality product.
- Availability and follow up of written and controlled manufacturing procedure.
- Approval and record in case of deviation in follow up of written procedure.
- Condition and care of ceiling and walls.
- Control of waste materials.
- Action and care to clean the utensils, between usage, used in the manufacturing process.
- Control to avoid cross-contamination during batch change over or product change over.
- Packaging / Labeling and shipping
- Adequacy marking system on the shipping containers.
- Use of approved packaging, labeling material.
- Use of approved packing materials.Control of printed materials.Adequacy of facilities to store the released materials awaiting shipment.
- Control on cleaning and use of appropriate tanker, trucks and storage tanks.
- Document control
- Control of specifications, test methods and other documents.
- Provision to record and to notify the clients of changes in specification or process.
- Quality Control
- Availability and follow up of written specifications and validated testing procedure.
- Adequacy of technical/experienced/qualified staff members.
- Sampling procedure of materials.Adequacy of instruments, their maintenance and periodic calibration.
- Adequacy of test procedure. Control for use of approved materials only.
- Indication of the reason for rejected material and corrective action taken to prevent the reoccurrence of problem.
- Control program for reagents to assure continued potency. (i.e. Date of received, date of opening, use before date, proper labeling)
- Preservation and evaluation of Laboratory standards.
- Control of reference sample and retain sample.
- Facilities in general
- Prohibition for smoking and eating in the area where necessary.
- Restriction for entry to production area to unauthorized person.
- Availability of appropriate toilet facility.
- Prohibition for jewelry and cosmetics in production area.
- Administrative
- Adequate support from engineering, production and quality control to meets our requirements.
- Whether or not the facility appears to be operating in such a manner that a shut or sale of the operation is imminent.
- Whether or not there appears to be an acceptable quality control department capable of making decision without repercussions.
- Previous Audit Report (if any)
- The previous audit report shall be verified for the Non Compliance Report and the closing of its observations. This shall also be the checklist for the coming audits.
- In some case ( if imported material is using or incase of difficult to audit manufacturers site) manufacturer are not qualified by carrying out site audit, QA Head shall send the check list to the respective manufacturer and get back duly filled and authorized by the respective manufacturers.
- Manufacturer’s also qualified by applying such system, those who are supplying material to the RKL since long and in no case material has been rejected or any difficulty faced during production/packing of the process.
- Frequency
- Approved Vendor for raw material shall be re-qualify after 03 years ± 03 months and approved vendor for packaging material shall be re-qualify after 05 years ± 05 months.
- After getting all the information along with the all related documents from vendor QA shall prepare the vendor risk assessment as per Annexure- IX.
- Adequacy of building and Facilities.
ABBREVIATIONS
- COA – Certificate of analysis
- NCR – Non Compliance Report
- QC – Quality Control
- QA – Quality Assurance
- GMO – Genetically Modified Organism
- TSE – Transmissible Spongiform Encephalopathy
- BSE – Bovine Spongiform Encephalopathy
- REFERENCES
- Active pharmaceutical ingredients committee (APIC) supplier qualification & management guideline
- The International Pharmaceutical Excipients Council Pharmaceutical Quality Group Joint GMP audit guideline for pharmaceutical excipients
- ANNEXURES
- Annexure-I : Approved Vendor List of Raw Materials
- Annexure-II : Approved Vendor List of Packing Materials
- Annexure-III : Vendor Assessment Checklist (For Raw Material)
- Annexure-IV : Vendor Assessment Checklist (For Packing Material)
- Annexure-V : Vendor Audit Report for Raw material
- Annexure-VI : Vendor Audit Report for Packing material
- Annexure-VII : Quality Agreement
- Annexure-VIII : Vendor Audit Schedule/Planner
- Annexure-IX : Vendor Risk Assessment
- Annexure-X : Questionnaire for Screening of TSE/BSE Risk
- Annexure-XI : Auditor Evaluation Form
- Annexure-XII : List of Certified Auditors
- DISTRIBUTION DETAILS
- Control Copy shall be distributed in Quality Assurance, Quality Control, Purchase and Warehouse.
- REVISION HISTORY
| SOP Version No. | Change Control No. | Reason For Revision | Effective Date |
