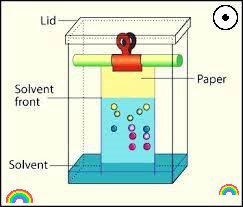
Chromatography in pharmaceuticals is a separation technique used to separate, identify, and quantify components of a mixture in order to ensure the quality, safety, and efficacy of pharmaceutical products. Chromatography involves the separation of a sample into its individual components based on their physicochemical properties using a stationary phase and a mobile phase. The stationary phase can be solid or liquid, and the mobile phase is a liquid or a gas that moves through the stationary phase carrying the sample components with it.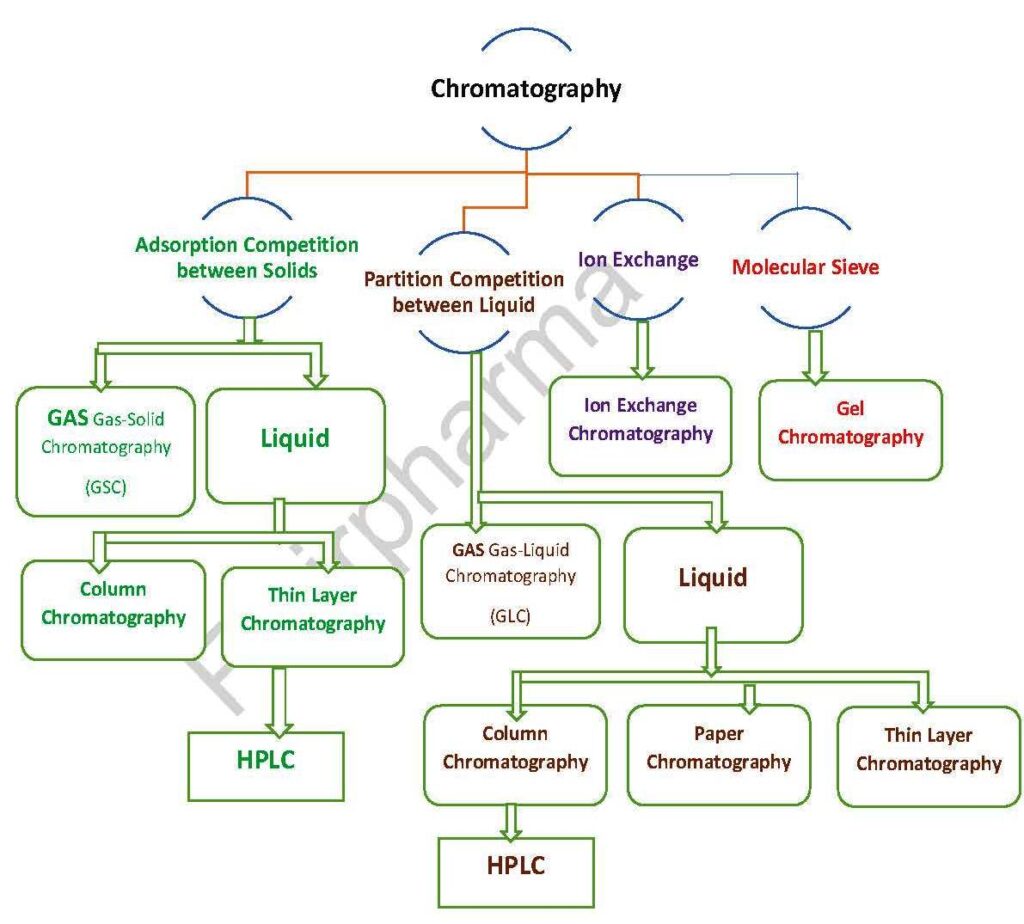
In pharmaceuticals, chromatogrphy is used for various purposes, including drug discovery, drug development, quality control, and regulatory compliance. It is commonly used for the analysis of raw materials, intermediates, finished products, and impurities in pharmaceuticals. Chromatogrphy techniques such as high-performance liquid chromatogrphy (HPLC), gas chromatography (GC), and thin-layer chromatogrphy (TLC) are widely used in the pharmaceutical industry.
Chromatogrphy in pharmaceuticals allows for the separation and identification of different components in a complex mixture, which is important for determining the purity of drugs, quantifying active pharmaceutical ingredients (APIs) and impurities, characterizing drug degradation products, and evaluating the stability of pharmaceutical formulations. It also plays a crucial role in ensuring compliance with regulatory requirements and standards set by regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the approval and release of pharmaceutical products to the market.
History of chromatography:
In 1906 Tswett used chromatography to separate plant pigments. He named the new technique chromatography because the analysis result was “written in color” along the length of the adsorbent column. Color is represented by chroma, and writing is represented by graphene.
The principles of chromatography in pharmaceuticals
The principles of chromatogrphy in pharmaceuticals are based on the separation of components in a sample mixture using a stationary phase and a mobile phase. The sample mixture is applied to the stationary phase, and the mobile phase is passed through the stationary phase, carrying the components with it at different rates based on their physicochemical properties. The principles of chromatogrphy in Quality Controls of pharmaceuticals include:
- Selectivity: The ability of the stationary phase to selectively retain or separate components in the sample based on their physicochemical properties such as size, charge, polarity, and affinity. The selectivity of the stationary phase is crucial in achieving desired separation and identification of components in pharmaceutical samples.
- Retention: The degree to which components in the sample interact with the stationary phase and are retained, or delayed, in their movement through the chromatogrphic system. Retention is influenced by the nature of the stationary phase, mobile phase composition, and physicochemical properties of the components.
- Elution: The process of components being washed or eluted from the stationary phase by the mobile phase, leading to their separation based on their differential interaction with the stationary phase. Elution is controlled by factors such as mobile phase flow rate, mobile phase composition, and column temperature.
- Detection: The ability to detect and quantify components as they elute from the chromatographic system. Detection techniques such as UV/Vis spectroscopy, mass spectrometry, and refractive index detection are commonly used in pharmaceutical chromatogrphy for the identification and quantification of components.
- Calibration: The establishment of a relationship between the concentration of a component in a sample and its response in the detection system. Calibration is essential for the accurate quantification of components in pharmaceutical samples.
- Validation: The process of demonstrating that the chromatogrphic method is suitable for its intended purpose, ensuring reliability, reproducibility, and accuracy of results. Validation is an important aspect of chromatogrphy in pharmaceuticals to meet regulatory requirements and ensure the quality of pharmaceutical products.
By leveraging these principles, chromatogrphy is widely used in pharmaceuticals for various applications such as drug discovery, development, quality control, and regulatory compliance, playing a critical role in ensuring the safety, efficacy, and quality of pharmaceutical products.
chromatography definition:
Chromatogrphy is a scientific technique used to separate, identify, and analyze the components of a mixture based on their different physical and/or chemical properties. It involves the distribution of components between two phases: a stationary phase and a mobile phase. The stationary phase can be a solid or liquid, and the mobile phase is typically a liquid or a gas. As the mixture is carried through the stationary phase by the mobile phase, the components of the mixture interact differently with the stationary phase, leading to their separation.
The separation is based on the differential affinity of the components of the mixture for the stationary and mobile phases and their distribution between the two phases. Components that have a higher affinity for the stationary phase will move more slowly through the column, while components that have a higher affinity for the mobile phase will move more quickly. This differential movement results in the separation of the components of the mixture into distinct bands or peaks, which can be detected and quantified to determine the composition of the mixture.
Chromatogrphy is a widely used technique in various fields such as pharmaceuticals, forensics, environmental science, food and beverage, chemistry, and biochemistry, among others. It can be performed using different types of chromatographic methods, such as gas chromatogrphy (GC), liquid chromatography (LC), ion exchange chromatography, size exclusion chromatogrphy, and many others, each with their specific principles and applications.
chromatography is categorized based on the mobile phase:
-
Liquid chromatography: mobile phase is a liquid. (LLC, LSC).
-
Gas chromatography: mobile phase is a gas. (GSC, GLC).
Classification based on packing of the stationary phase:
- Thin layer chromatogrphy (TLC): the stationary phase is a thin layer supported on glass, plastic or aluminium plates.
- Paper chromatogrphy (PC): the stationary phase is a thin film of liquid supported on an inert support.
- Column chromatogrphy (CC): stationary phase is packed in a glass column.
Separation classification based on the force of separation:
- Adsorption chromatogrphy.
- Partition chromatogrphy.
- Ion exchange chromatogrphy.
- Gel filtration chromatogrphy.
- Affinity chromatogrphy.
-
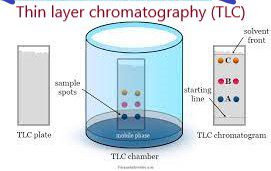
Thin layer chromatography (TLC):
- This technique is used to determine what components are present and how pure a compound is. TLC is a practical method because it is relatively quick and uses little material. In TLC, a mixture of two or more compounds is divided between a stationary phase and a mobile phase.
- Stationary phase: a thin layer of adsorbent (usually silica gel or alumina) coated on a plate.
- Mobile phase: is a developing liquid that travels up the stationary phase, carrying the samples with it.
-
Paper Chromatography:
A chromatogrphic partitioning technique that uses filter paper strips as a carrier or inert support. The partition between two immiscible phases is what determines how mixtures of solutes separate on filter paper. One is typically water that has adhered to the paper’s cellulose fibers (stationary phase). The organic solvent then moves past the sample on the paper in the second step (stationary phase). The stationary aqueous phase bound by the cellulose separates from the mobile phase.
General Procedure:
-
- Choice of paper and solvent to be used.
- Desalting of the sample.
- Application of the sample.
- Equilibration of paper.
- Identification of substances.
-
Chromatography paper
Chromatogrphy paper, also known as filter paper or chromatographic paper, is a type of paper that is specially designed for use in chromatogrphy. It is a porous, absorbent paper made from high-quality cellulose fibers that are treated to create a consistent and uniform surface for chromatographic separations.
Chromatogrphy paper is typically made from materials such as cotton, cellulose, or glass fiber, and is available in various thicknesses and pore sizes to suit different chromatographic applications. The paper is typically white or colorless, and it may be coated with a thin layer of adsorbent material, such as silica or cellulose, to enhance its separation capabilities.
Chromatogrphy paper is used as the stationary phase in paper chromatogaphy, which is a type of chromatography where the sample mixture is applied to the paper, and the separation is achieved by the differential migration of components in the sample as the mobile phase (solvent) moves through the paper via capillary action. The components in the sample mixture migrate at different rates on the chromatogrphy paper based on their physicochemical properties, resulting in the separation and visualization of the individual components.
Chromatogrphy paper is commonly used in various applications, including qualitative and quantitative analysis of chemical compounds, separation and identification of organic and inorganic compounds, analysis of food and beverages, environmental monitoring, and forensic analysis. It is a cost-effective and versatile medium for performing chromatogrphic separations, especially for small-scale experiments and educational purposes.
-
Columnar Chromatography (CC):
- This includes chromatogrphic methods in which: The stationary phase is packed into a column. The mobile phase is a moving liquid or gas. According to the mechanism of separation of solutes, five significant types of CC are distinguished. Usually, one mechanism predominates but does not exclude the others
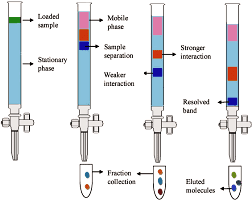
- Column Chromatography: Column chromatogrphy Stationary phase is held in a narrow tube through which the mobile phase is forced under pressure or under the effect of gravity.
-
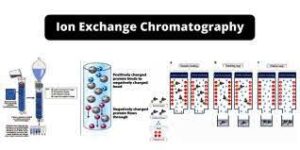
Ion exchange chromatography
- Ion exchange chromatogrphy is a type of chromatographic technique used in quality control laboratories to separate and analyze charged molecules based on their affinity for charged stationary phases. It is widely used in pharmaceutical, biotechnology, food and beverage, and other industries to ensure the quality and purity of products.
- In ion exchange chromatography, a sample containing charged molecules (ions) is passed through a column packed with a stationary phase that has charged functional groups. The stationary phase may be either positively charged (cation exchange chromatography) or negatively charged (anion exchange chromatography). The charged molecules in the sample interact with the stationary phase through electrostatic forces, leading to their separation based on their charge and affinity for the stationary phase.
- The basic principle of ion exchange chromatography is that molecules with opposite charges to the stationary phase will bind to the column, while molecules with similar charges will pass through more quickly. By controlling the composition of the mobile phase (i.e., the buffer solution) and adjusting the pH and ionic strength, the analyst can manipulate the interaction between the sample ions and the stationary phase to achieve the desired separation.
- Ion exchange chromatogrphy is commonly used in quality control laboratories for tasks such as determining the purity of pharmaceuticals, monitoring the levels of ions in food and beverages, analyzing water quality, and assessing the performance of ion exchange resins used in industrial processes. It is a powerful and versatile technique that allows for the precise separation and quantification of charged molecules, making it an essential tool in quality control for various industries.
The critical parameters of chromatography:
The critical parameters of chromatography are key variables or factors that significantly impact the separation performance and efficiency of a chromatographic method. These parameters may vary depending on the specific type of chromatography being used, such as gas chromatography (GC), liquid chromatography (LC), or other forms of chromatography. Some of the critical parameters of chromatography include:
- Stationary Phase: The type, composition, and properties of the stationary phase, which is the solid or liquid material that lines the chromatography column and interacts with the analytes being separated. The choice of stationary phase can greatly influence the selectivity, retention, and separation efficiency of the chromatographic method.
- Mobile Phase: The type, composition, and properties of the mobile phase, which is the liquid or gas that carries the analytes through the chromatography column. The mobile phase composition, flow rate, and temperature can affect the retention, elution, and separation efficiency of analytes.
- Column Dimensions: The dimensions of the chromatography column, such as length, diameter, and particle size (for packed columns), or inner diameter and length (for capillary columns in gas chromatography). Column dimensions can affect the separation efficiency, resolution, and analysis time of a chromatographic method.
- Temperature: The temperature at which the chromatographic separation is performed, which can influence the retention, elution, and selectivity of analytes. Temperature is particularly important in gas chromatography, where it affects the volatility and partitioning of analytes between the stationary phase and mobile phase.
- Flow Rate: The rate at which the mobile phase is pumped through the chromatography column, which can affect the retention, elution, and peak shape of analytes. The flow rate needs to be optimized to balance the need for efficient separation with the limitations of column pressure and analysis time.
- Detector Settings: The settings and parameters of the detector used to detect and quantify the separated analytes, such as detector type, wavelength (for UV/Vis or fluorescence detectors), temperature (for thermal conductivity or flame ionization detectors in gas chromatography), and sensitivity. The detector settings can impact the sensitivity, selectivity, and accuracy of the chromatographic analysis.
- Sample Preparation: The preparation and pretreatment of the sample being analyzed, including factors such as sample matrix, sample volume, sample concentration, and sample injection volume. Proper sample preparation is critical for obtaining accurate and reliable results in chromatographic analysis.
- Analyte Properties: The physicochemical properties of the analytes being separated, such as their polarity, size, charge, and volatility. Analyte properties can affect their interaction with the stationary phase and mobile phase, and therefore impact the chromatographic separation.
- Method Validation: The validation of chromatographic method to ensure its reliability, accuracy, and reproducibility. Method validation includes parameters such as accuracy, precision, linearity, the limit of detection, the limit of quantitation, and robustness. Proper method validation is essential to ensure the quality and validity of the chromatographic results.
Optimizing these critical parameters in chromatography is important to achieve the desired separation performance, resolution, and sensitivity in a chromatographic analysis. Depending on the specific chromatographic technique and the analytes being analyzed, other parameters may also be critical and need to be carefully controlled and optimized for optimal results.
Frequently Asked Questions about Chromatography.
What is chromatography?
Answer: Chromatogrphy is a separation technique used to separate, identify, and quantify components in a mixture based on their physicochemical properties using a stationary phase and a mobile phase.
Why is chromatography important in pharmaceuticals?
Answer: Chromatogrphy is important in pharmaceuticals as it enables the separation, identification, and quantification of components in drug samples, ensuring the quality, safety, and efficacy of pharmaceutical products. It is used in drug development, quality control, impurity analysis, and regulatory compliance.
What are the common types of chromatography used in pharmaceuticals?
Answer: Common types of chromatogrphy used in pharmaceuticals include high-performance liquid chromatography (HPLC), gas chromatography (GC), thin-layer chromatogrphy (TLC), and liquid chromatography-mass spectrometry (LC-MS), among others.
How is chromatography used in drug development?
Answer: Chromatogrphy plays a crucial role in drug development by analyzing drug candidates, determining their purity, quantifying active pharmaceutical ingredients (APIs), characterizing drug degradation products, and evaluating the stability of pharmaceutical formulations.
What is the role of chromatography in the quality control of pharmaceuticals?
Answer: Chromatogrphy is extensively used in the quality control of pharmaceuticals to ensure the consistency and quality of raw materials, intermediates, finished products, and impurities. It allows for the analysis and quantification of components, monitoring of batch-to-batch variations, and compliance with regulatory requirements.
What are the advantages of using chromatography in pharmaceutical analysis?
Answer: The advantages of using chromatogrphy in the pharmaceutical analysis include high separation efficiency, sensitivity, specificity, versatility, and reproducibility. Chromatography also allows for the analysis of complex mixtures, small sample volumes, and a wide range of physicochemical properties.
How is chromatography validated in pharmaceutical analysis?
Answer: Chromatogrphy methods used in the pharmaceutical analysis must be validated to ensure their reliability, reproducibility, and accuracy. Validation typically involves demonstrating parameters such as specificity, linearity, accuracy, precision, robustness, and system suitability, following regulatory guidelines.
What are the challenges in chromatography in pharmaceuticals?
Answer: Challenges in chromatogrphy in pharmaceuticals include method development for complex samples, optimization of separation conditions, selection of appropriate stationary and mobile phases, matrix interference, detection sensitivity, calibration accuracy, and compliance with regulatory requirements.
Can chromatography detect impurities in pharmaceuticals?
Answer: Yes, chromatogrphy is commonly used for the detection and quantification of impurities in pharmaceuticals, including process-related impurities, degradation products, and residual solvents. It is crucial in ensuring the safety and efficacy of pharmaceutical products.
What are the regulatory requirements for chromatography in pharmaceutical analysis?
Answer: Regulatory requirements for chromatogrphy in pharmaceutical analysis vary by region, but typically include compliance with guidelines from regulatory agencies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which provide guidance on method validation, system suitability, and data integrity, among others.