Atomic absorption spectroscopy (AAS) is an analytical technique that measures the concentration of specific elements in a sample by passing a beam of light through the sample and measuring how much of that light is absorbed by the atoms of the element of interest. AAS is widely used in environmental testing, clinical analysis, and industrial quality control due to its sensitivity and versatility.
Atomic absorption spectroscopy (AAS) is an analytical technique used to measure the concentration of specific elements in a sample. It works by passing a beam of light through a sample, and measuring how much of that light is absorbed by the atoms of the element of interest.
AAS can be used to analyze a wide range of sample types, including liquids, solids, and gases. It is particularly useful for analyzing the concentration of metals in a sample, such as lead in water, or iron in blood.
The basic components of an AAS instrument include a light source, a sample holder, a monochromator, a detector, and a computer for data analysis. The light source is typically a hollow cathode lamp that emits light at a specific wavelength that is absorbed by the atoms of the element being analyzed.
AAS is a highly sensitive technique, capable of detecting trace amounts of elements in a sample. It is widely used in environmental testing, clinical analysis, and industrial quality control.
Principle of Atomic Absorption Spectroscopy
The principle of atomic absorption spectroscopy (AAS) is based on the fact that each element has a unique electronic structure, which results in the absorption of light at specific wavelengths.
In AAS, a beam of light, typically generated by a hollow cathode lamp, is passed through a sample containing the element of interest. The atoms in the sample absorb the light at specific wavelengths, which corresponds to the energy required to promote electrons from the ground state to an excited state.
The amount of light absorbed by the atoms is proportional to the concentration of the element in the sample. A detector measures the intensity of the transmitted light, which is used to calculate the concentration of the element in the sample.
A monochromator is used to isolate the specific wavelength of light that corresponds to the absorption of the element being analyzed while eliminating other interfering wavelengths.
The sensitivity and accuracy of AAS can be improved by using a flame, graphite furnace, or other atomizers to convert the sample into a gaseous form, which enhances the interaction between the light and the atoms.
Process
Atomic absorption spectroscopy (AAS) involves several steps to perform. Here’s a general overview of the process:
- Sample preparation: The sample is typically dissolved in an appropriate solvent and filtered to remove any particulates that could interfere with the analysis. For solid samples, they may need to be digested or ashed before dissolving in a solvent.
- Atomization: The sample is introduced into an atomizer, which converts the sample into a gaseous state for analysis. The most common types of atomizers used in AAS are flame and graphite furnace atomizers.
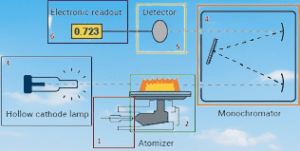
- Light source: A hollow cathode lamp is used to generate a beam of light that is specific to the element being analyzed. The light is directed through the atomized sample.
- Wavelength selection: A monochromator is used to select the specific wavelength of light that corresponds to the absorption of the element being analyzed while eliminating other interfering wavelengths.
- Detector: A detector measures the intensity of the transmitted light, which is used to calculate the concentration of the element in the sample.
- Calibration: A series of standard solutions of known concentration are used to generate a calibration curve that relates the intensity of the transmitted light to the concentration of the element being analyzed.
- Analysis: The sample is analyzed by comparing its absorption to the calibration curve. The concentration of the element in the sample can be calculated based on the curve and the amount of sample analyzed.
- Data interpretation: The results of the analysis are typically reported in units of concentration, such as parts per million (ppm) or parts per billion (ppb).
It’s worth noting that the specific details of AAS can vary depending on the type of sample being analyzed, the instrument being used, and the specific requirements of the analysis.
More articles to read :
UV Spectrophotometer Principle
FTIR spectroscopy (Fourier Transform Infrared Spectroscopy)
Application of Atomic Absorption Spectroscopy
| Application | Element of Interest | Sample Type |
|---|---|---|
| Environmental Testing | Lead, Cadmium, Arsenic, Mercury, Copper, Zinc | Water, Soil, Air |
| Clinical Analysis | Iron, Magnesium, Calcium, Sodium, Potassium | Blood, Urine |
| Industrial Quality Control | Iron, Copper, Zinc, Nickel, Chromium, Aluminum | Metals, Alloys, Plastics |
| Food and Beverage Testing | Sodium, Potassium, Calcium, Magnesium, Iron, Copper, Zinc | Milk, Wine, Beer, Fruit Juice, Cereals, Vegetables |
| Forensic Analysis | Lead, Barium, Antimony, Strontium | Soil, Paint, Gunshot Residue |
| Agricultural Testing | Potassium, Nitrogen, Phosphorus, Calcium, Magnesium | Soil, Plant Tissue, Fertilizers |
Instrumentation of atomic absorption spectroscopy
The basic instrumentation of an atomic absorption spectroscopy (AAS) instrument includes the following components:
- Light source: A hollow cathode lamp (HCL) is typically used to generate a beam of light that is specific to the element being analyzed. The HCL consists of a cathode made of the element of interest, a gas-filled bulb, and an anode. When a voltage is applied to the lamp, a discharge is generated, which produces the specific spectral line for the element being analyzed.
- Sample introduction: The sample is introduced into the instrument using a variety of techniques depending on the type of sample and the specific requirements of the analysis. The most common techniques are flame and graphite furnace atomization, but other techniques such as cold vapor atomization, hydride generation, and electrothermal vaporization can also be used.
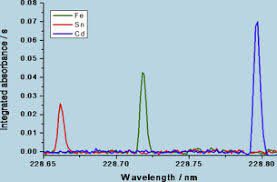
- Monochromator: A monochromator is used to isolate the specific wavelength of light that corresponds to the absorption of the element being analyzed while eliminating other interfering wavelengths. The monochromator typically consists of a diffraction grating or prism that separates the different wavelengths of light and a slit that selects a specific wavelength to pass through to the detector.
- Detector: A detector measures the intensity of the transmitted light, which is used to calculate the concentration of the element in the sample. The most common types of detectors used in AAS are photomultiplier tubes (PMTs), which convert the photons of light into electrical signals that can be amplified and measured.
- Data analysis: The output from the detector is typically processed by a computer, which calculates the concentration of the element in the sample based on the intensity of the transmitted light and the calibration curve generated from standards of known concentration.
- Accessories: Various accessories can be added to the basic AAS instrument to enhance its capabilities, such as autosamplers for high throughput analysis, furnace cooling systems for improved furnace atomization, and flow injection systems for automated sample introduction.
The specific details of an AAS instrument can vary depending on the manufacturer and model, but the basic components and principles are the same.
Flame atomic absorption spectroscopy
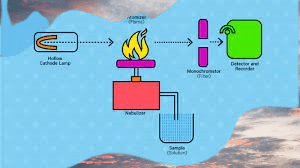
Flame atomic absorption spectroscopy (FAAS) is a common technique used in atomic absorption spectroscopy (AAS) for the quantitative analysis of metal ions in a sample. In FAAS, the sample is introduced into a flame where it is atomized and excited by the heat of the flame, and the resulting absorption is measured to determine the concentration of the metal ion.
The basic steps involved in FAAS are:
- Sample preparation: The sample is typically dissolved in an appropriate solvent and filtered to remove any particulates that could interfere with the analysis. For solid samples, they may need to be digested or ashed before dissolving in a solvent.
- Sample introduction: The sample is introduced into the instrument using a nebulizer, which produces a fine mist of the sample solution that is then carried into the flame by a stream of inert gas, typically argon.
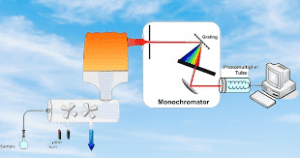
- Flame atomization: The nebulized sample is introduced into the flame, which is typically fueled by acetylene gas and air or nitrous oxide. The heat of the flame atomizes and excites the metal ions in the sample.
- Light source: A hollow cathode lamp is used to generate a beam of light that is specific to the element being analyzed. The light is directed through the atomized sample in the flame.
- Wavelength selection: A monochromator is used to select the specific wavelength of light that corresponds to the absorption of the element being analyzed while eliminating other interfering wavelengths.
- Detector: A detector measures the intensity of the transmitted light, which is used to calculate the concentration of the element in the sample.
- Calibration: A series of standard solutions of known concentration are used to generate a calibration curve that relates the intensity of the transmitted light to the concentration of the element being analyzed.
- Analysis: The sample is analyzed by comparing its absorption to the calibration curve. The concentration of the element in the sample can be calculated based on the curve and the amount of sample analyzed.
FAAS is a widely used technique due to its relative simplicity and low cost compared to other AAS techniques. However, it does have some limitations, such as interference from other elements in the sample matrix and the potential for flame instability.
Graphite furnace atomic absorption spectroscopy
Graphite furnace atomic absorption spectroscopy (GFAAS) is another technique used in atomic absorption spectroscopy (AAS) for the quantitative analysis of metal ions in a sample. Unlike flame atomic absorption spectroscopy (FAAS), GFAAS uses a graphite furnace to atomize and excite the sample, allowing for higher sensitivity and lower detection limits.
The basic steps involved in GFAAS are:
- Sample preparation: The sample is typically dissolved in an appropriate solvent and filtered to remove any particulates that could interfere with the analysis. For solid samples, they may need to be digested or ashed before dissolving in a solvent.
- Sample introduction: The sample is introduced into the graphite furnace using a micro syringe or autosampler, typically in volumes of microliters or nanoliters.
- Graphite furnace atomization: The furnace is heated in a series of steps to dry and ash the sample, and then atomize and excite the metal ions. The temperature is carefully controlled to prevent degradation of the sample and ensure optimal atomization and excitation.
- Light source: A hollow cathode lamp is used to generate a beam of light that is specific to the element being analyzed. The light is directed through the atomized sample in the graphite furnace.
- Wavelength selection: A monochromator is used to select the specific wavelength of light that corresponds to the absorption of the element being analyzed, while eliminating other interfering wavelengths.
- Detector: A detector measures the intensity of the transmitted light, which is used to calculate the concentration of the element in the sample.
- Calibration: A series of standard solutions of known concentration are used to generate a calibration curve that relates the intensity of the transmitted light to the concentration of the element being analyzed.
- Analysis: The sample is analyzed by comparing its absorption to the calibration curve. The concentration of the element in the sample can be calculated based on the curve and the amount of sample analyzed.
GFAAS offers several advantages over FAAS, such as higher sensitivity, lower detection limits, and reduced interference from other elements in the sample matrix. However, it also has some limitations, such as the potential for sample matrix effects and the need for careful temperature control during the atomization process.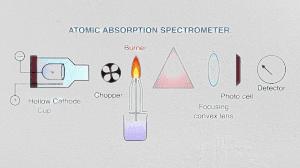
Use of the Atomic Absorption Spectroscopy
| Application | Description |
|---|---|
| Metal impurity analysis in raw materials | AAS can be used to analyze metal impurities in raw materials used for drug manufacturing, such as excipients, active pharmaceutical ingredients, and packaging materials. |
| Dissolution testing | Cleaning validation is a process used to ensure that equipment used in drug manufacturing is free from residual drugs and cleaning agents. AAS can be used to detect and quantify metal residues from the equipment surface. |
| Cleaning validation | Cleaning validation is a process used to ensure that equipment used in drug manufacturing is free from residual drug and cleaning agents. AAS can be used to detect and quantify metal residues from the equipment surface. |
| Stability testing | Stability testing is used to evaluate the stability of a drug product over time. AAS can be used to monitor the stability of metal ions in a drug product, which may affect its quality and safety. |
| Water testing | AAS can be used to analyze the concentration of metal ions in water used for drug manufacturing, cleaning, and testing. High levels of metal ions may affect the quality and safety of the drug product. |
| Elemental impurity testing | Elemental impurity testing is a requirement under the International Council for Harmonisation (ICH) guidelines for pharmaceuticals. AAS can be used to quantify elemental impurities, such as heavy metals, in drug products. |
| Bioanalysis | AAS can be used to measure metal ions in biological samples, such as blood, urine, and tissue, to evaluate drug toxicity and exposure. |
Frequently Asked Questions
What is atomic absorption spectroscopy?
Answer: Atomic absorption spectroscopy (AAS) is a technique used to determine the concentration of metal ions in a sample by measuring the absorption of light at a specific wavelength. AAS can be performed using flame or graphite furnace atomization.
How does atomic absorption spectroscopy work?
Answer: AAS works by atomizing and exciting the metal ions in a sample using a flame or graphite furnace, and then measuring the absorption of light at a specific wavelength using a hollow cathode lamp and monochromator. The concentration of the metal ions can be determined by comparing the absorption to a calibration curve generated from standard solutions of known concentration.
What is the difference between flame and graphite furnace atomic absorption spectroscopy?
Answer: Flame atomic absorption spectroscopy (FAAS) and graphite furnace atomic absorption spectroscopy (GFAAS) differ in the method of atomization and excitation. FAAS uses a flame to atomize and excite the sample, while GFAAS uses a graphite furnace. GFAAS offers higher sensitivity and lower detection limits than FAAS.
What are the advantages of atomic absorption spectroscopy?
Answer: AAS offers several advantages, including high sensitivity, selectivity, and accuracy for the determination of metal ions in a sample. It is also a relatively simple and cost-effective technique compared to other analytical methods.
What are the limitations of atomic absorption spectroscopy?
Answer: AAS has some limitations, such as interference from other elements in the sample matrix, limited dynamic range, and the need for sample preparation. It may also require calibration for each element of interest.
What is a hollow cathode lamp?
Answer: A hollow cathode lamp is a type of light source used in AAS to generate a beam of light at a specific wavelength that corresponds to the absorption of the element being analyzed. The lamp contains a cathode made of the element of interest, which is ionized by a high-voltage discharge and emits light at a characteristic wavelength.
What is a monochromator?
Answer: A monochromator is an optical device used in AAS to select a specific wavelength of light that corresponds to the absorption of the element being analyzed. It uses a diffraction grating or prism to disperse the light into its component wavelengths and then selects the desired wavelength.
What is the range of concentrations that can be analyzed using atomic absorption spectroscopy?
Answer: The range of concentrations that can be analyzed using AAS depends on several factors, including the sensitivity of the instrument, the linearity of the calibration curve, and the dynamic range of the detector. Generally, AAS can detect concentrations in the parts-per-billion to parts-per-million range.
What is the role of the sample matrix in atomic absorption spectroscopy?
Answer: The sample matrix can affect the accuracy and precision of AAS results by causing interference with the atomization and excitation of the metal ions. Matrix effects can be reduced by adjusting the sample preparation or using a matrix-matched calibration curve.
What is the role of quality control in atomic absorption spectroscopy?
Answer: Quality control is important in AAS to ensure the accuracy and precision of the results. Quality control measures may include running standards and blanks, verifying calibration curves, and assessing instrument performance.
You may also read about Microbiology Guidelines

I’ve been surfing on-line greater than 3 hours lately, yet I never found any fascinating article like yours. It’s pretty worth enough for me. In my view, if all site owners and bloggers made good content as you did, the internet will probably be much more helpful than ever before.