The UV Spectrophotometer principle is based on the measurement of the interaction between ultraviolet light and a sample. It uses the Beer-Lambert law to determine the concentration of absorbing substances in a sample by measuring the absorption of UV light at a specific wavelength. This technique is widely used in various fields, including chemistry, biochemistry, pharmaceuticals, and environmental monitoring.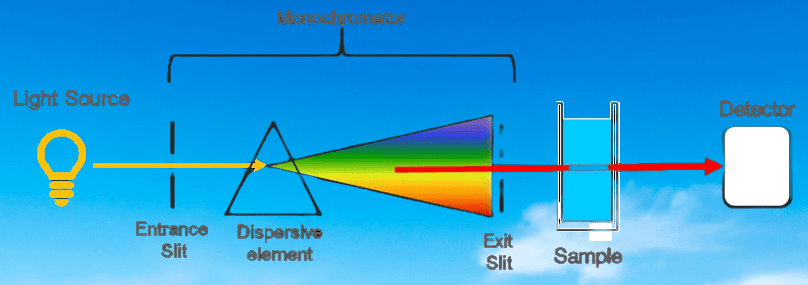
A UV spectrophotometer is a scientific instrument used to measure the absorption of ultraviolet (UV) light by a sample. It is widely used in chemistry, biochemistry, and other fields to analyze and identify molecules based on their absorption spectra. UV spectrophotometers are commonly used in quality control, research, and development laboratories to quantitatively measure the amount of a specific substance in a sample, such as a drug or protein. They can also be used to measure the purity of a sample, identify impurities, and monitor chemical reactions. Overall, UV spectrophotometers are a powerful tool for analyzing and characterizing molecules in a wide range of applications.
Principle of UV Spectrophotometer:
The basic principle of a UV spectrophotometer is to measure the absorbance of ultraviolet (UV) light by a sample. In a quality control lab, a UV spectrophotometer is used to determine the concentration of a specific substance in a sample, such as a drug or protein.
The instrument works by passing a beam of UV light through the sample, and measuring how much of the light is absorbed by the sample at different wavelengths.
Amount of light absorbed α concentration of the substance in the sample.
The instrument typically consists of a light source, a sample holder, a monochromator to select specific wavelengths of light, a detector to measure the amount of light transmitted through the sample, and a display to show the results.
To use a UV spectrophotometer in a quality control lab, a standard curve is first established by measuring the absorbance of known concentrations of the substance of interest. The absorbance of the unknown sample is then measured, and the concentration is determined by comparing it to the standard curve.
Overall, the UV spectrophotometer is a powerful tool for quantitative analysis in quality control labs, providing accurate and reliable measurements of the concentration of specific substances in a wide range of samples.
The law equation of UV Spectrophotometer is the Beer-Lambert Law, which is given by:
A = ɛcl
where A is the absorbance of light by a sample, ɛ is the molar absorptivity or extinction coefficient, c is the concentration of the absorbing species in the sample, and l is the path length of light through the sample. This equation describes the relationship between the concentration of an absorbing substance in a sample, the path length of the light through the sample, and the amount of light absorbed by the sample. By measuring the absorbance of a sample at a specific wavelength, the concentration of the absorbing substance can be determined using the Beer-Lambert Law.
Main Parts of UV Spectrophotometer:
The main parts of a UV spectrophotometer are as follows:
- Light Source: It is the source of the ultraviolet radiation used in the analysis. Typically, the source is a deuterium (D2) lamp or a xenon (Xe) lamp that produces a broad spectrum of ultraviolet light.
- Monochromator: It is an optical device that selects a specific wavelength of light from the broad spectrum produced by the light source. The monochromator typically consists of a diffraction grating or a prism that disperses the light into its component wavelengths, and a slit that allows only a specific wavelength to pass through.
- Sample Holder: It is a device that holds the sample in place and allows the light to pass through it. The sample holder can be a cuvette or a cell made of quartz or glass.
- Detector: It is a device that measures the intensity of the light that passes through the sample. The most common detector used in UV spectrophotometers is a photomultiplier tube (PMT), which converts the light into an electrical signal that can be measured.
- Electronics: It includes the circuitry that controls the operation of the instrument, such as the power supply, amplifier, and data acquisition system. The electronics are responsible for controlling the light source, monochromator, and detector, and for converting the detector signal into a usable format.
- Display: It is a device that shows the results of the analysis. The display can be a simple analog meter, a digital readout, or a computer monitor.
Overall, the instrumentation of a UV spectrophotometer is designed to select a specific wavelength of ultraviolet light, pass it through a sample, and measure the intensity of the light that passes through the sample. This allows for quantitative analysis of the amount of a specific substance in the sample.

Applications of the UV spectroscopy of Quality Control Lab.
UV spectroscopy has a wide range of applications in many fields, including chemistry, biochemistry, pharmaceuticals, and environmental monitoring. Some of the common applications of UV spectroscopy are:
-
- Quantitative Analysis: UV spectroscopy is commonly used for quantitative analysis of various compounds, such as proteins, nucleic acids, and drugs. It is used to determine the concentration of these compounds in a sample by measuring the absorption of UV light at a specific wavelength.
- Qualitative Analysis: UV spectroscopy is also used for qualitative analysis, such as identifying the presence of a specific compound in a sample. The absorption spectrum of a compound is unique and can be used to identify the compound based on its characteristic absorption peaks.
- Purity Analysis: UV spectroscopy is used to determine the purity of a sample by measuring the absorbance of UV light at specific wavelengths. The absorbance ratio at two different wavelengths can provide information about the purity of a compound and can be used to identify the presence of impurities in a sample.
- Kinetics Studies: UV spectroscopy is used to monitor chemical reactions by measuring changes in the absorbance of UV light over time. This allows for the study of reaction kinetics and reaction mechanisms.
- Environmental Monitoring: UV spectroscopy monitors environmental pollutants, such as pesticides and polycyclic aromatic hydrocarbons (PAHs), in water and air samples.
- Material Science: UV spectroscopy can be used to study the properties of materials, such as the electronic structure and optical properties of semiconductors and polymers.
Overall, UV spectroscopy is a versatile analytical tool that has many applications in various fields, making it an essential technique for quality control, research, and development
Double-beam UV spectrophotometer
A double-beam UV spectrophotometer is a type of UV spectrophotometer that uses two separate light beams to measure the absorption of light by a sample. One beam is passed through the sample, while the other beam is passed through a reference or blank solution. By measuring the difference in the absorption of the two beams, the absorbance of the sample can be accurately determined, eliminating errors due to fluctuations in the light source or detector.
In a double-beam UV spectrophotometer, the light source is typically a deuterium lamp that emits light in the ultraviolet range and a tungsten-halogen lamp that emits light in the visible range. The two beams of light are split using a beam splitter, and each beam is passed through a monochromator to select the desired wavelength of light. The sample and reference solutions are placed in separate cuvettes and placed in the path of the two beams. The light passing through each cuvette is detected by a photodiode detector, and the absorbance of the sample is determined by measuring the difference in the detected light intensity between the sample and reference beams.
Double-beam UV spectrophotometers offer several advantages over single-beam instruments, including higher accuracy and precision, increased sensitivity, and the ability to correct fluctuations in the light source and detector. They are commonly used in various applications, including quantitative analysis, purity analysis, kinetics studies, and environmental monitoring.
Frequently Asked Questions:
What is UV spectroscopy?
Answer: UV spectroscopy is a technique used to analyze the interaction of ultraviolet light with a sample to determine the presence and concentration of specific compounds.
What is the principle of UV spectroscopy?
Answer: The principle of UV spectroscopy is based on the Beer-Lambert law, which states that the amount of light absorbed by a sample is directly proportional to the concentration of the absorbing substance in the sample and the path length of the light through the sample.
What are the applications of UV spectroscopy?
Answer: UV spectroscopy is used in various fields such as chemistry, biochemistry, pharmaceuticals, environmental monitoring, and material science. It can be used for quantitative and qualitative analysis, purity analysis, kinetics studies, and environmental monitoring.
What are the main parts of a UV spectrophotometer?
Answer: The main parts of a UV spectrophotometer are the light source, monochromator, sample holder, detector, electronics, and display.
What is the wavelength range of UV spectroscopy?
Answer: The wavelength range of UV spectroscopy is typically between 190 and 400 nm.
What is the difference between UV and visible spectroscopy?
Answer: UV spectroscopy measures the absorption of light in the ultraviolet region, whereas visible spectroscopy measures the absorption of light in the visible region.
What are the advantages of using UV spectroscopy?
Answer: The advantages of using UV spectroscopy include its high sensitivity, non-destructive nature, and the ability to analyze a wide range of samples.
What is the importance of calibration curves in UV spectroscopy?
Answer: Calibration curves are important in UV spectroscopy because they allow for the determination of the concentration of a substance in a sample based on its absorbance at a specific wavelength.

What are the limitations of UV spectroscopy?
Answer: The limitations of UV spectroscopy include interference from other compounds, limited accuracy in complex mixtures, and a narrow wavelength range.
How is UV spectroscopy used in pharmaceutical analysis?
Answer: UV spectroscopy is commonly used in pharmaceutical analysis for the quantitative and qualitative analysis of drugs and their degradation products, as well as for impurity profiling and stability

1 thought on “UV Spectrophotometer Principle 2023”