A dispensing booth is crucial in the pharmaceutical industry, providing a controlled environment for accurate and safe dispensing of pharmaceutical ingredients and materials. Discover how dispensing booths ensure operator protection, prevent cross-contamination and maintain product integrity. Explore their features, benefits, and applications in pharmaceutical manufacturing.
Working Principle of dispensing booth
The working principle of a dispensing booth involves creating a controlled environment that ensures operator safety, minimizes the risk of cross-contamination, and maintains product integrity during the dispensing process. Here’s a breakdown of the working principle:
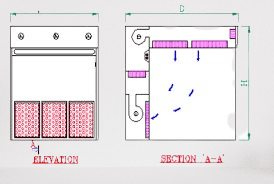
- Enclosed Workspace: A dispensing booth is designed as an enclosed workspace with walls, a ceiling, and a work surface that provides a physical barrier between the operator and the surrounding environment.
- Airflow Control: The dispensing booth incorporates an air handling system, typically including a ventilation system and filters, to control the airflow within the booth. The airflow is carefully designed to prevent contaminants from entering the booth and to protect the operator.
- HEPA Filtration: High-Efficiency Particulate Air (HEPA) filters are integral to the airflow system of the dispensing booth. These filters effectively capture and remove airborne particles, including dust, microorganisms, and other contaminants, ensuring a clean and controlled environment.
- Airflow Direction: Dispensing booths usually maintain a unidirectional airflow pattern. Clean, filtered air is supplied from the top of the booth and directed toward the operator, creating a protective barrier of clean air between the operator and the work area.
- Containment and Exhaust: The airflow system ensures the containment of any particles or contaminants generated during the dispensing process. The contaminated air is then exhausted through appropriate filters and ventilation systems, preventing it from re-entering the booth or spreading to other areas.
- Operator Protection: The dispensing booth is designed to shield the operator from potential exposure to hazardous or sensitive materials. It provides a physical barrier and a controlled airflow environment that minimizes the risk of inhalation or contact with the dispensed substances.
- Validation and Monitoring: Regular verification and monitoring of the dispensing booth’s airflow velocity, pressure differentials, filter efficiency, and cleanliness levels are performed to ensure compliance with regulatory standards and maintain the integrity of the controlled environment.
By implementing these principles, dispensing booths provide a safe and controlled workspace for pharmaceutical professionals to measure and dispense ingredients accurately, ensuring product quality and operator safety throughout the dispensing process.
Differential Pressure limit for dispensing booth
The specific differential pressure limit for a dispensing booth can vary depending on various factors, including regulatory guidelines, facility requirements, and the type of substances being handled. However, a commonly used and recommended differential pressure limit for a dispensing booth is typically set at around 15-25 Pascal (Pa).
The purpose of maintaining a differential pressure in a dispensing booth is to ensure proper containment of potentially hazardous or sensitive materials within the booth and prevent their release into the surrounding environment. The differential pressure is the difference in pressure between the inside of the booth (positive pressure) and the surrounding area (negative pressure).
By maintaining a slightly higher pressure inside the booth compared to the surrounding area, any potential leakage or escape of contaminants is minimized. The positive pressure helps prevent contaminants from entering the booth, providing an additional layer of protection for the operator and maintaining the integrity of the dispensing process.
It’s important to note that the specific differential pressure limit for a dispensing booth may be subject to specific regulations or industry standards in different regions. Therefore, it’s recommended to consult relevant guidelines, such as those from regulatory authorities or international standards organizations, to determine the appropriate differential pressure limit for a dispensing booth in your specific jurisdiction.
Applications of Dispensing Booths in Pharmaceuticals.
Dispensing booths have various applications in the pharmaceutical industry, primarily in the areas of material handling, compounding, and weighing processes. Here are some common applications of dispensing booths in pharmaceuticals:
- Active Pharmaceutical Ingredient (API) Dispensing: Dispensing booths are used for the accurate and controlled dispensing of APIs, ensuring precise measurements and preventing cross-contamination between different ingredients.
- Excipient Dispensing: Excipients, which are inactive substances used as carriers or fillers in pharmaceutical formulations, can be dispensed in a controlled environment to maintain their purity and prevent contamination.
- Powder and Granule Handling: Dispensing booths are utilized for handling powders and granules, such as in the preparation of solid dosage forms. They ensure operator safety and prevent the dispersion of airborne particles during the weighing and dispensing process.
- Sterile Product Compounding: In sterile compounding areas, dispensing booths provide a controlled and clean environment for compounding sterile medications, including aseptic filling and reconstitution processes.
- Nutraceutical and Dietary Supplement Manufacturing: Dispensing booths are used in the production of nutraceuticals and dietary supplements to ensure accurate weighing and dispensing of ingredients and maintain product quality.
- Quality Control and Sampling: Dispensing booths can be employed in quality control laboratories for accurately dispensing samples and reference materials during testing and analysis.
- Hazardous Material Handling: For the handling of hazardous or toxic substances, such as cytotoxic drugs or potent compounds, dispense booths offer operator protection and containment, minimizing the risk of exposure.
- Research and Development: In pharmaceutical research and development facilities, dispense booths are used for the precise weighing and dispensing of experimental compounds and formulations.
- Customized Formulations: Dispense booths provide a controlled environment for the preparation of customized formulations, such as personalized medications or specialized dosage forms.
By utilizingdispense booths in these applications, pharmaceutical companies can ensure accurate measurements, maintain product integrity, prevent cross-contamination, and enhance operator safety during various material handling and compounding processes.
Dispensing booth specifications:
| Technical Aspect | Description |
|---|---|
| Purpose | Provides a controlled environment for accurate and safe dispensing of pharmaceutical materials |
| Airflow Velocity | Typically 0.45 to 0.65 m/s (90 to 130 fpm) |
| Filters | High-Efficiency Particulate Air (HEPA) filters |
| Filter Replacement | As per the manufacturer’s recommendations or regular maintenance schedules |
| Differential Pressure | Recommended range: 15-25 Pascal (Pa) |
| Particle Containment | Achieved through unidirectional airflow, HEPA filtration, physical barriers, and sealing |
| Customization | Can be customized to specific requirements, such as glove ports, adjustable work surfaces |
| Performance Validation | Airflow velocity testing, particle count analysis, containment testing, filter integrity |
| Integration with Cleanroom | Can be integrated with other cleanroom equipment and systems |
| Regulatory Guidelines | Compliance with FDA, ISO, and local health authority guidelines |
Frequently Asked Questions:
What is the purpose of a dispensing booth?
Answer: The purpose of a dispense booth is to provide a controlled environment for accurate and safe dispensing of pharmaceutical ingredients and materials. It ensures operator protection, prevents cross-contamination, and maintains product integrity.
What is the recommended airflow velocity in a dispensing booth?
Answer: The recommended airflow velocity in a dispense booth is typically in the range of 0.45 to 0.65 meters per second (m/s) or 90 to 130 feet per minute (fpm). This velocity helps maintain a barrier of clean air between the operator and the work area, preventing the ingress of contaminants.
What type of filters are used in dispensing booths?
Answer: dispense booths commonly use High-Efficiency Particulate Air (HEPA) filters. These filters have a high efficiency in capturing and removing airborne particles, including dust, microorganisms, and other contaminants, to maintain a clean and controlled environment.
How often should the filters in a dispensing booth be replaced?
Answer: The frequency of filter replacement depends on factors such as the type and quantity of materials being dispensed, environmental conditions, and regulatory requirements. Generally, filters should be replaced according to the manufacturer’s recommendations or during regular maintenance schedules to ensure optimal performance.
What is the recommended differential pressure in a dispensing booth?
Answer: The recommended differential pressure for a dispense booth is typically set at around 15-25 Pascal (Pa). This differential pressure helps maintain containment within the booth, preventing the release of contaminants into the surrounding environment.
How is the containment of particles achieved in a dispensing booth?
Answer: Containment of particles in a dispense booth is achieved through a combination of factors, including unidirectional airflow, HEPA filtration, physical barriers, and proper sealing of the booth. These measures ensure that particles generated during the dispensing process are captured, contained, and safely exhausted from the booth.
Are dispensing booths customizable to specific requirements?
Answer: Yes, dispense booths can be customized to meet specific requirements. They can be designed and configured to accommodate different sizes, material handling needs, and levels of operator protection. Customizations may include features like glove ports, adjustable work surfaces, lighting, and ergonomic considerations.
How is the performance of a dispensing booth validated?
Answer: The performance of a dispense booth is validated through various tests and measurements, including airflow velocity testing, particle count analysis, containment testing, and filter integrity testing. These validations ensure that the booth meets the required specifications and regulatory standards.
Can dispensing booths be integrated with other cleanroom equipment?
Answer: Yes, dispense booths can be integrated with other cleanroom equipment and systems, such as pass-through hatches, airlocks, and HVAC (Heating, Ventilation, and Air Conditioning) systems, to create a comprehensive and controlled environment for material handling and processing.
What are the regulatory guidelines for dispensing booth design and usage?
Answer: Regulatory guidelines for dispense booth design and usage can vary depending on the region and specific industry regulations. Common guidelines include those from organizations such as the FDA (Food and Drug Administration), ISO (International Organization for Standardization), and local health authorities. It’s important to consult relevant regulations and standards applicable to your jurisdiction.
