Reverse Laminar Air Flow (RLAF) is a specialized air handling system used in the pharmaceutical industry to maintain a controlled and contaminant-free environment. By directing airflow from a contaminated area towards a clean area, RLAF prevents the introduction of contaminants into critical zones, ensuring the safety and quality of pharmaceutical products. Reverse Laminar Air Flow (RLAF), or Reverse Laminar Flow (RLF), is a specialized air handling system used in pharmaceutical and cleanroom environments. It is designed to maintain a controlled environment by preventing the contamination of sensitive products, equipment, or processes.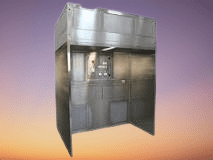
Unlike traditional laminar airflow systems, where the air flows from a clean area toward a contaminated area, RLAF operates in the opposite direction. The air flows from a contaminated area towards a clean area, minimizing the risk of contaminant introduction.
RLAF is commonly employed in sterile manufacturing areas such as aseptic processing, filling lines, and compounding areas in a pharmaceutical setting. It provides a high level of product protection by ensuring that any contaminants generated within the process are contained and directed away from critical areas.
The key features and benefits of RLAF in the pharmaceutical industry.
- Contamination control: RLAF creates a controlled airflow pattern that prevents contaminants from reaching critical areas where sterile products are handled or manufactured. It helps maintain the required cleanliness levels by minimizing the entry of particulate matter, microorganisms, and other sources of contamination.
- Operator protection: RLAF systems also provide protection to the operators by creating a barrier between them and potentially hazardous substances or processes. The reverse airflow helps prevent exposure to harmful aerosols, dust, or vapors.
- Cross-contamination prevention: RLAF minimizes the risk of cross-contamination between different processes or product lines. Directing the airflow away from clean areas reduces the chances of contamination transfer from one place to another.
- Enhanced product quality: By maintaining a clean environment and preventing contamination, RLAF contributes to pharmaceutical products’ overall quality and integrity. It helps ensure compliance with regulatory standards and reduces the likelihood of product recalls or failures.
- Validation and monitoring: RLAF systems require validation and ongoing monitoring to ensure their effectiveness. Regular airflow velocity measurements, particle counts, and microbial sampling are performed to confirm that the system is operating within specified parameters and meeting the required standards.
It’s worth noting that RLAF is just one of the various technologies and strategies used in the pharmaceutical industry to maintain clean and controlled environments. Other systems, such as High-Efficiency Particulate Air (HEPA) filters, airlocks, and stringent cleaning protocols, are often integrated with RLAF to achieve the desired level of contamination control.
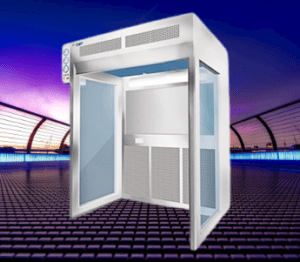
Reverse Laminar air flow principle
The working principle of Reverse Laminar Air Flow (RLAF) involves the controlled direction of airflow from a contaminated area towards a clean area, ensuring that contaminants are contained and prevented from reaching critical zones. Here’s a breakdown of the working principle:
- Airflow Direction: In RLAF, the airflow is reversed compared to traditional laminar flow systems. Instead of flowing from a clean area toward a contaminated area, the air is directed from a contaminated area toward a clean area.
- Contaminated Area: The contaminated area refers to the region where potentially harmful substances, particles, or processes are present. This can include areas with active pharmaceutical ingredient (API) handling, equipment cleaning, or other processes that generate contaminants.
- Clean Area: The clean area is the designated zone where sensitive pharmaceutical products, equipment, or processes requiring a controlled environment are located. It could be an aseptic filling line, sterile compounding area, or any other critical zone.
- Airflow Pattern: RLAF creates a controlled airflow pattern that effectively prevents contaminants from entering the clean area. The airflow is typically generated by ventilation systems, air handlers, and HEPA (High-Efficiency Particulate Air) filters.
- Containment and Exhaust: The RLAF system ensures that the contaminated air is contained within the contaminated area and exhausts safely. This prevents the spread of contaminants to the clean area and maintains the required cleanliness levels.
- Air Filtration: RLAF systems incorporate HEPA filters that effectively capture and remove airborne particles and microorganisms. These filters have high efficiency in removing particulate matter, typically with an efficiency of 99.97% for particles as small as 0.3 micrometers.
- Validation and Monitoring: Regular validation and monitoring of the RLAF system are crucial to ensuring its effectiveness. Airflow velocity measurements, particle counts, and microbial sampling are performed to verify that the system is operating within specified parameters and meeting regulatory standards.
By utilizing the reverse airflow principle, RLAF minimizes the risk of contamination, protects sensitive products and processes, and maintains a controlled environment in pharmaceutical settings.

Difference between Reverse Laminar Airflow and Laminar Air Flow
| Aspect | RLAF | LAF |
|---|---|---|
| Airflow Direction | From contaminated area to clean area | From clean area to contaminated area |
| Contaminant Containment | Contaminants are contained and directed away | Contaminants are pushed away from critical areas |
| Purpose | Protect clean areas from contamination | Protect clean areas from contamination |
| Contaminant Sources | Typically used for processes with active | Generally used for processes with low |
| contamination sources or hazardous materials | contamination sources or non-hazardous materials | |
| Airflow Pattern | Reverse airflow pattern with exhaust | Unidirectional airflow pattern with exhaust |
| Operator Protection | Provides barrier against hazardous substances | Provides limited operator protection |
| Cross-Contamination Risk | Low risk of cross-contamination due to | Higher risk of cross-contamination due to |
| airflow direction and containment | airflow direction | |
| Air Filter Efficiency | Utilizes HEPA filters with high efficiency | Utilizes HEPA filters with high efficiency |
| Validation and Monitoring | Requires regular validation and monitoring | Requires regular validation and monitoring |
| Application | Aseptic processing, filling lines, compounding | Laboratories, medical facilities, cleanrooms, |
| areas in pharmaceutical manufacturing | research facilities |
It’s important to note that RLAF, Reverse Laminar Air Flow is a specialized airflow system used in specific scenarios where the reverse airflow direction provides enhanced protection and containment of contaminants. LAF, on the other hand, is a more commonly used airflow system in various cleanroom applications.
Major components of the RLAF
The major components of a Reverse Laminar Air Flow (RLAF) system typically include the following:
- Contaminated Area: This refers to the specific zone or area where potentially harmful substances or processes are present. It could include areas where active pharmaceutical ingredient (API) handling, equipment cleaning, or other processes generate contaminants.
- Clean Area: The clean area is the designated zone where sensitive pharmaceutical products, equipment, or processes requiring a controlled environment are located. This area needs to be protected from contamination.
- Air Handling Units (AHUs): AHUs are responsible for conditioning and circulating the air within the RLAF, Reverse Laminar Air Flow system. They regulate temperature, humidity, and airflow velocity to meet the required specifications of the clean area.
- HEPA Filters: High-Efficiency Particulate Air (HEPA) filters are critical components in Reverse Laminar Air Flow, RLAF systems. These filters have a high efficiency in capturing and removing airborne particles, including microorganisms, to maintain the desired cleanliness levels.
- Airflow Control Devices: These devices, such as dampers and louvers, help control and direct the airflow within the RLAF, Reverse Laminar Air Flow system. They ensure that the airflow pattern remains consistent and in the desired direction.
- Exhaust System: The exhaust system is responsible for safely removing the contaminated air from the RLAF system and discharging it to the appropriate ventilation or exhaust system.
- Monitoring and Control Systems: These systems include sensors, monitors, and control panels that continuously monitor and regulate parameters such as airflow velocity, pressure differentials, temperature, humidity, and filter performance. They ensure that the RLAF system operates within specified parameters and can alert operators to any deviations or issues.
- Validation Ports: Validation ports provide access points for conducting airflow velocity measurements, particle counts, and microbial sampling to validate the effectiveness of the RLAF, Reverse Laminar Air Flow system. These ports allow for regular monitoring and verification of the system’s performance.
It’s important to note that the specific components and their configurations can vary depending on the design and requirements of the RLAF, Reverse Laminar Air Flow system in a particular facility. Customizations may be made based on the size of the clean area, the level of contamination risk, and other specific factors.
Frequently Asked Questions:
What is Reverse Laminar Air Flow (RLAF)?
Answer: RLAF, Reverse Laminar Air Flow is a specialized air handling system used in pharmaceutical and cleanroom environments where the airflow is directed from a contaminated area towards a clean area, preventing the introduction of contaminants into critical zones.
What are the benefits of RLAF in pharmaceutical settings?
Answer: RLAF, Reverse Laminar Air Flow provides contamination control, operator protection, prevention of cross-contamination, enhanced product quality, and adherence to regulatory standards.
How does RLAF differ from traditional Laminar Air Flow (LAF)?
Answer: The key difference lies in the airflow direction. RLAF, Reverse Laminar Air Flow directs airflow from a contaminated area to a clean area, while LAF directs airflow from a clean area to a contaminated area.
What types of areas in pharmaceutical manufacturing use RLAF?
Answer: RLAF, Reverse Laminar Air Flow is commonly used in sterile manufacturing areas such as aseptic processing, filling lines, compounding areas, and other critical zones where product integrity and cleanliness are paramount.
How does RLAF prevent contamination?
Answer: RLAF creates a controlled airflow pattern that prevents contaminants generated within the process from reaching clean areas, minimizing the risk of contamination.
Are there any specific regulations or guidelines for RLAF in the pharmaceutical industry?
Answer: Various regulatory bodies, such as the FDA (Food and Drug Administration) and international standards like ISO 14644, provide guidelines and requirements for cleanroom environments, including Reverse Laminar Air Flow RLAF systems.
How is the effectiveness of RLAF validated and monitored?
Answer: Regular validation and monitoring of Reverse Laminar Air Flow RLAF systems are performed, including airflow velocity measurements, particle counts, and microbial sampling, to ensure compliance with specified parameters and standards.
Can RLAF eliminate all types of contaminants?
Answer: While RLAF is highly effective in controlling airborne contaminants, it is essential to implement other measures, such as proper gowning, cleaning protocols, and equipment sterilization, to address other potential sources of contamination.
Can RLAF be retrofitted into existing cleanroom facilities?
Answer: Yes, Reverse Laminar Air Flow systems can be retrofitted into existing facilities, but it requires careful planning and design considerations to integrate the reverse airflow pattern effectively.
How important is the maintenance of RLAF systems?
Answer: Regular maintenance, including filter replacement, cleaning of ventilation components, and airflow testing, is crucial to ensuring the continued effectiveness of Reverse Laminar Air Flow systems and maintaining a controlled environment.

Ralf capcity
RLAF capacity depend on the area to be cater under the RLAF. After volume calculation CFM will calculate & design the RLAF.
This post on the Reverse Laminar Air Flow principle was incredibly insightful! I appreciate the detailed explanation of how RLAF improves contamination control in cleanroom environments. It’s fascinating to see how these innovations are shaping the future of pharmaceutical manufacturing. Thanks for sharing!
This post on the Reverse Laminar Air Flow principle is incredibly informative! I appreciate how you broke down the technical details and explained the significance of RLAF in a clear way. It’s fascinating to see how this technology improves lab environments. Thank you for sharing this valuable knowledge!
Great overview of the Reverse Laminar Air Flow principle! I appreciated the clear explanation of its applications and benefits in maintaining sterility. It’s fascinating to see how advancements in air flow technology continue to enhance safety in pharmaceutical settings. Looking forward to more insights from Flair Pharma!
This blog post on the Reverse Laminar Air Flow (RLAF) Principle is incredibly informative! I appreciate how you broke down the complexities of air flow principles in such an accessible way. It’s fascinating to see how RLAF can enhance safety in various environments. Looking forward to more insights from Flair Pharma!