Performance Qualification Protocol for Purified Water Generation & Distribution System (Phase-1), This Performance Qualification Protocol (Phase-1) for the Purified Water Generation & Distribution System in a pharmaceutical plant outlines the comprehensive procedures, responsibilities, and methodologies required to ensure the system consistently produces purified water meeting specified quality standards. The document details the scope, pre-qualification requirements, sampling plans, testing procedures, and acceptance criteria necessary to validate the system’s performance, ensuring compliance with industry regulations and standards.
DETAILS OF EQUIPMENT/SYSTEM
- DEPARTMENT: Engineering
- STAGE OF QUALIFICATION: PERFORMANCE QUALIFICATION (PHASE-1)
- LOCATION: Utilities
- MAKE: Watermass Systems
- EQUIPMENT ID: [To be filled]
Table of Contents of PQ
- Protocol Pre-Approval
- Objective
- Scope
- Responsibility
- Equipment/System Description
- Equipment/Materials Required for Qualification
- Methodology
- Qualification Results & Recording of Observations
- Requalification Criteria
- Details of Discrepancies
- Summary/Conclusion
- References
- Abbreviations
- Annexures
Protocol Pre-Approval
Signing of approval page of this document indicates the agreement of Qualification approach described in this document. If any modification approach becomes necessary, a revision through change control shall be prepared, checked, and approved. This document cannot be executed unless approved.
Approvals
- Prepared by: [Name, Designation, Department, Signature/Date]
- Reviewed by: [Name, Designation, Department, Signature/Date]
- Approved by: [Name, Designation, Department, Signature/Date]
Objective
The objective of this Performance Qualification Protocol (Phase-I) is to provide a high degree of assurance and reliability about the performance of the Purified Water Generation & Distribution System at [Company Name]. The Phase-1 Qualification aims to:
- Integrate procedures, personnel, systems, and materials needed for the operation of the water system.
- Demonstrate that the system can consistently meet the water quality & quantity requirements as specified.
- Provide rigorous testing to demonstrate the effectiveness and reproducibility of the total integrated process.
- Establish and confirm that the entire system will operate within specified operating ranges.
- Develop and evaluate system operation, sanitization, and maintenance procedures.
- Verify that the water produced and delivered to the points of use consistently meets the required quality attributes and acceptance criteria in line with the intended design.
Scope in PQ
The scope of this protocol is to provide the procedure and guidelines for the Performance Qualification Phase-1 of the Purified Water Generation & Distribution System. The Qualification shall be performed after the successful completion and authorization of Installation and Operational Qualification.
Responsibility in PQ
Engineering
- Review the protocol and undergo training on methodology.
- Execute the qualification activity and record data as per the outlined procedures.
- Review compiled data and ensure it meets the acceptance criteria.
QC & Microbiology
- Review the protocol and undergo training on methodology.
- Sample predefined user and sampling points as per procedures.
- Analyze water samples, compile results, and release findings.
- Trend identified quality attributes.
Quality Assurance
- Prepare the protocol and train cross-functional departments.
- Monitor qualification activities.
- Compile analytical and system-related data and prepare the report.
- Approve the report and ensure compliance with the protocol.
Equipment/System Description
The Water Treatment System will treat water to achieve the required quality and quantity of Purified Water as per the agreed design criteria. The Purified Water System consists of the following sections:
- Chlorination with Sodium hypochlorite solution for disinfection.
- Multi-grade filtration for removing suspended solids and turbidity.
- Softening to remove dissolved ions responsible for hardness.
- Micron Cartridge Filter for removing suspended solids.
- Ultrafiltration to reduce SDI and remove bacteria, colloidal silica, and turbidity.
- UV System to treat microbiologically unsafe water.
- Anti-scalent dosing to reduce scaling tendency.
- pH correction dosing.
- De-chlorination with Sodium Meta Bi-Sulphate.
- High Salinity Reverse Osmosis (HSRO-I) for removing dissolved impurities.
- Electro De-ionization (EDI) for polishing the water.
- Storage and distribution of purified water to various user points.
Equipment/Materials Required for Qualification PQ
- Sampling tools as per QC & Microbiology procedures.
- SDI kit.
Pre-Requisite for PQ
- Training: The validation task force (QA, QC, Microbiology, & Engineering) must be trained on the validation concept. Training records shall be attached as Attachment-1.
- Identification of Executors: Personnel involved in protocol execution shall be trained and recorded in Annexure-I “Identification of Executors”.
- Verification of IQ & OQ completion: Confirm the completion of IQ and OQ with respect to observation recording and summary report.
- Calibration Status: Verify the calibration status of instruments and devices used in the qualification process.
Methodology of PQ
The Performance Qualification will be conducted in three phases:
Phase I
- Establish appropriate operating ranges.
- Sampling from EDI onwards in the treatment process and from each point of use.
- Test incoming feed water for compliance with specifications.
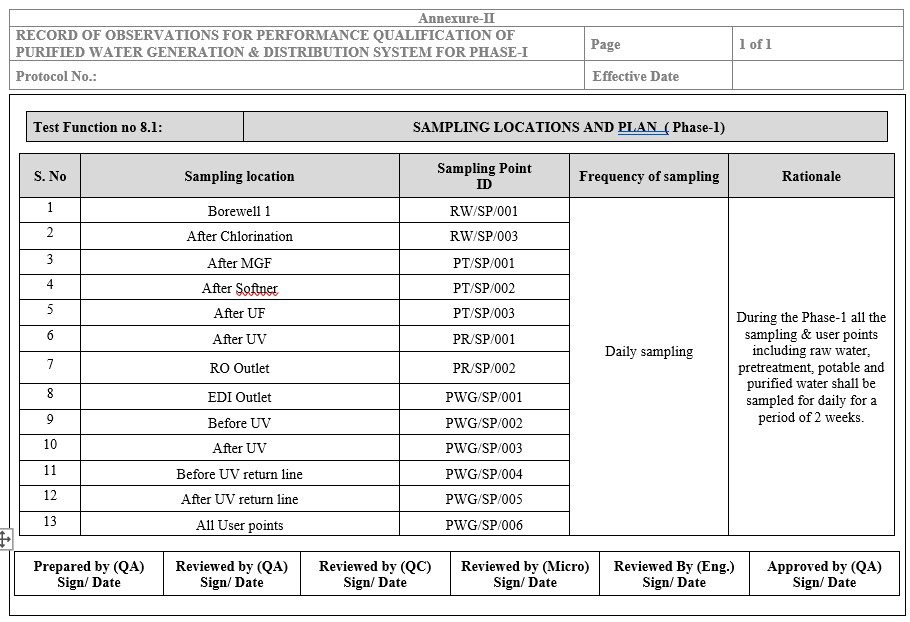
- Sampling plan detailed in Annexure-II.
- Establish provisional alert and action limits.
- Determine sanitization frequency and regeneration steps.
Phase II
- Demonstrate consistent operation within established ranges.
- Confirm the production and delivery of water meeting required quality and quantity.
- Finalize sanitization and regeneration frequency.
Phase III
- Verify extended performance over one year.
- Ensure seasonal variations are evaluated and treated.
Sampling Locations and Plan in PQ
Details of sampling/user points and their frequencies are provided in Annexure for Phase-I.
Performance Qualification Protocol for Purified Water Generation & Distribution System (Phase-1)
Performance Qualification of Phase-I
Objective
Establish appropriate operating ranges and provide data for finalizing operating, sanitization, and maintenance procedures.
Procedure
- Conduct Phase-I Qualification for 14 days.
- Operate, regenerate, and sanitize the Purified Water System as per SOPs.
- Collect samples from all user & sampling points as per the plan in Annexure-II.
- Analyze samples as specified in Annexure-III.
- Record data in ATDS (Analytical Data Sheets) and summarize in the final report.
Qualification Results & Recording of Observations
All analytical data shall be recorded as per the respective specifications in QC Chemical & Microbiology ATDS.
Requalification Criteria
Requalification is required if there are major changes or modifications in the system that impact the qualification state or if there are excursions impacting quality.
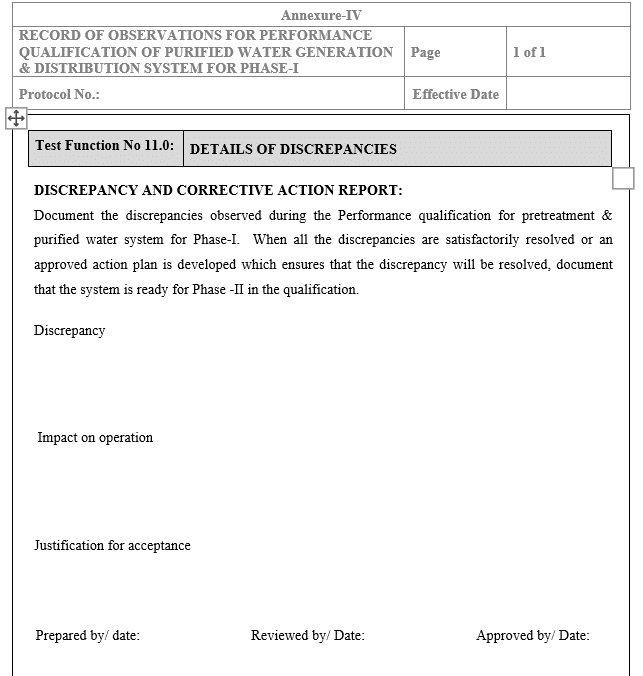
Details of Discrepancies
Report discrepancies in Annexure-IV ‘Discrepancy & Corrective Action Report’. Ensure corrective actions are satisfactorily completed.
Summary/Conclusion
Compile and review all test functions and verify the resolution of any discrepancies or deviations. Performance Qualification of Phase-1 is acceptable when all conditions specified are met.
References
- Design Documents.
- WHO Technical Report Series No. 929, 2005
- ISPE Baseline Guidelines for Water & Steam.
- USP General Chapter Water for Pharmaceutical Use <1231>.
- USFDA ITG: Water for Pharmaceutical Use.
Abbreviations
- ITG: Initial Terminal Guidance
- RO: Reverse Osmosis
- MGF: Multi-Grade Filter
- QA: Quality Assurance
- QC: Quality Control
- SOP: Standard Operating Procedure
- GTP: General Testing Procedure
- STP: Standard Testing Procedure
- IQ: Installation Qualification
- OQ: Operational Qualification
- PQ: Performance Qualification
- SDI: Slit Density Index
- ATDS: Analytical Data Sheets
- VMP: Validation Master Plan
- WHO: World Health Organization
- ISPE: International Society of Pharmaceutical Engineers
Annexures
- Annexure I: Pre-requisites
- Annexure II: Sampling Locations and Plan
- Annexure III: Critical Quality Points (Sampling & User points) with identified Quality attributes.
- Annexure IV: Discrepancy & Corrective Action Report

Annexure-III
| RECORD OF OBSERVATIONS FOR PERFORMANCE QUALIFICATION OF PURIFIED WATER GENERATION & DISTRIBUTION SYSTEM FOR PHASE-I |
| Description | A Clear, Colourless liquid. |
| pH | 6.5 to 8.5 |
| Chlorides | NMT 250ppm |
| Sulphate | NMT 200ppm |
| Nitrates | NMT 45 ppm |
| Total Dissolved Solids | NMT 500 ppm |
| Total Suspended Solids | NMT 500 ppm |
| Total hardness | NMT 300 ppm |
| Total aerobic microbial Counts | For Information |
| Escherichia coli | Must be absent |
| Salmonella species | Must be absent |
| Pseudomonas aeruginosa | Must be absent |
| Staphylococcus aureus | Must be absent |
For Purified water Below Specifications Must match.
| Description | A Clear, Colourless liquid. |
| pH | Between 5.0 and 7.0 |
| Nitrates | NMT 0.2 ppm |
| Heavy metals | NMT 0.1 ppm |
| Total Organic Carbon | NMT 500 ppb |
| Conductivity | NMT 1.3 µS/cm |
| Total aerobic microbial Counts | NMT 100 CFU/mL |
| Escherichia coli | Must be absent |
| Salmonella | Must be absent |
| Pseudomonas aeruginosa | Must be absent |
| Staphylococcus aureus | Must be absent |
How to Monitor Quality of Water in pharmaceuticals.