Karl Fischer titrator (KFT) is a cornerstone analytical technique in the pharmaceutical industry, particularly within Quality Control (QC) laboratories, where precise water content determination is crucial. This method’s reliability and accuracy make it indispensable for ensuring product quality, stability, and compliance with regulatory standards.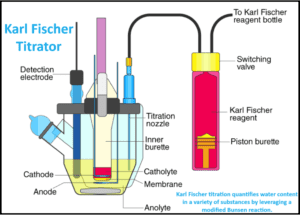
Basics of Karl Fischer Titrator
Karl Fischer titration quantifies water content in a variety of substances by leveraging a modified Bunsen reaction. This reaction involves the interaction of iodine and sulfur dioxide in an alcohol-based medium. Typically, methanol or diethylene glycol monoethyl ether (DEGEE) acts as the reactive alcohol, and modern reagents often use imidazole or primary amines instead of pyridine, which is a noxious carcinogen.
The core reaction can be summarized as:

Here, the alcohol (ROH) reacts with sulfur dioxide and a base (R’N) to form an intermediate alkylsulfite salt, which is then oxidized by iodine, consuming water in the process. The endpoint of the titration is detected when excess iodine is present, indicating all water has reacted.
Types of Karl Fischer Titration
There are two primary types of Karl Fischer titration: Volumetric and Coulometric, each suited to different water content ranges.
1. Volumetric KFT: In volumetric titration, iodine is mechanically added to the sample solution, and the water content is calculated based on the volume of reagent used. This method is effective for water content ranging from 100 ppm to 100%. It has two reagent systems:
- One-component reagents: Contain all necessary chemicals (iodine, sulfur dioxide, base) dissolved in an alcohol, making them easier to handle and less expensive.
- Two-component reagents: Separate the iodine and the remaining components, providing better stability and faster titration times but at a higher cost.

2. Coulometric KFT: Coulometric titration generates iodine electrochemically within the titration cell. This method is suitable for very low water content, ranging from 1 ppm to 5%. There are two types of coulometric cells:
- Fritted-cell (conventional): Uses a diaphragm to separate the anode and cathode, preventing iodine reduction at the cathode.
- Fritless-cell: Innovative design without a diaphragm, reducing maintenance and costs, and increasing automation suitability.
Application of Karl Fischer titrator in Pharmaceutical QC Labs
In pharmaceutical QC labs, ensuring the precise measurement of water content is critical for product stability and efficacy. Karl Fischer titrators play a crucial role in various stages of product development and manufacturing, from raw material assessment to final product testing.
Operational Workflow:
- Sample Preparation: Depending on the expected water content, samples are weighed and prepared for analysis.
- Titration: The sample is introduced into the titration cell, where either volumetric or coulometric titration is performed.
- Endpoint Detection: The titrator detects the endpoint using a double platinum pin indicator electrode.
- Calculation: The amount of water is calculated using the volume of reagent used (volumetric) or the total charge passed (coulometric).
Monitoring and Standards: Performance is regularly monitored using NIST traceable water standards in sealed ampoules or solid standards. These standards ensure the titrator’s accuracy and reliability over time.
Sample Size Considerations: The amount of sample used varies with the anticipated water content. For instance:
- For high water content (100%), 0.02 to 0.05 g is used in volumetric titration.
- For trace water content (1 ppm), 10 g or more may be required for coulometric titration.

Advantages and Limitations of Karl Fischer Titration
| Advantages | Limitations |
|---|---|
| High accuracy and precision in water content determination. | Sensitive to sample pH; requires buffering if outside optimal range (pH 5-8). |
| Suitable for a wide range of water content (1 ppm to 100%). | Reagents can be sensitive to light and moisture, requiring careful storage. |
| Can be used with solid, liquid, and gas samples. | Volumetric titration is less suitable for very low water content (below 100 ppm). |
| Coulometric method is ideal for low water content (1 ppm to 5%). | Coulometric titration has a lower upper limit for water content (up to 5%). |
| Fast and efficient, with relatively short analysis times. | Requires calibration and maintenance of electrodes. |
| Small sample sizes are sufficient for analysis, especially in coulometric titration. | Methanol, commonly used as a solvent, is toxic and requires careful handling. |
| Automation is possible, enhancing reproducibility and reducing operator error. | High initial cost for the titration apparatus. |
| Modern reagents are pyridine-free, making them safer and more environmentally friendly. | Interference from certain substances (e.g., ketones, aldehydes) may require special handling or pre-treatment. |
| Can be used to analyze complex matrices, including oils and fats. | The presence of other reducing or oxidizing substances can interfere with the titration results. |
| Offers direct measurement of water content without needing extensive sample preparation. | Karl Fischer reagents have limited shelf life and need regular replacement. |
Conclusion
Karl Fischer titration remains an essential analytical technique in pharmaceutical QC laboratories, providing precise and reliable water content measurement. Its adaptability to various sample types and water content ranges, along with advancements in reagent formulation and titration cell design, ensure it meets the rigorous demands of the pharmaceutical industry. As technology advances, Karl Fischer titration continues to evolve, offering even greater efficiency, accuracy, and ease of use.
Best manufacturers for Karl Fischer titration equipment:
| Manufacturer | Description |
|---|---|
| Metrohm | A leading manufacturer known for high-quality titration equipment, including both volumetric and coulometric Karl Fischer titrators. They offer comprehensive solutions with advanced automation and user-friendly software. |
| Mettler Toledo | Renowned for their precision instruments, Mettler Toledo offers a range of Karl Fischer titrators known for accuracy, robustness, and advanced features like touch screen interfaces and automated processes. |
| Hanna Instruments | Provides cost-effective and reliable Karl Fischer titrators suitable for various applications. They are known for their compact design and ease of use. |
| Thermo Fisher Scientific | Offers a variety of analytical instruments, including Karl Fischer titrators, known for their reliability and integration with other laboratory equipment. |
| HACH | Known for producing durable and accurate Karl Fischer titration equipment, HACH caters to a wide range of industries, including pharmaceuticals and water analysis. |
| G.R. Scientific | Specializes in Karl Fischer titration instruments and provides both volumetric and coulometric systems that are highly regarded for their precision and ease of use. |
| Schott Instruments (now part of SI Analytics) | Offers a variety of Karl Fischer titrators known for their high precision and innovative technology. They are well-regarded in the laboratory equipment industry. |
| Kyoto Electronics (KEM) | A Japanese manufacturer known for high-quality titration systems, including Karl Fischer titrators, which are popular for their accuracy and advanced features. |
| ECH Elektrochemie Halle | Specializes in innovative Karl Fischer titration systems with a focus on precision and user-friendly design, offering both volumetric and coulometric options. |
| Analytik Jena | Provides advanced analytical instruments, including Karl Fischer titrators, known for their high performance and integration with modern laboratory practices. |
Frequently Asked Questions:
What is Karl Fischer titration used for?
Answer: Karl Fischer titration is used for determining the water content in a wide variety of substances, including chemicals, pharmaceuticals, oils, and food products.
Which electrode is used in KF titration?
Answer: A double platinum pin indicator electrode is used in Karl Fischer titration to detect the endpoint.
How is the titer determined in Karl Fischer?
Answer: The titer in Karl Fischer titration is determined by titrating a known amount of water standard and calculating the amount of Karl Fischer reagent required to reach the endpoint.
What is the principle of KF?
Answer: The principle of Karl Fischer titration is based on the quantitative reaction of water with iodine and sulfur dioxide in the presence of an alcohol and a base, resulting in the formation of an alkylsulfate salt and hydriodic acid salt.
What is the KF factor limit?
Answer: The KF factor limit refers to the range of water content that can be accurately measured using Karl Fischer titration. For volumetric titration, it is typically from 100 ppm to 100%, and for coulometric titration, it is from 1 ppm to 5%.
What is the pH of KF titration?
Answer: The optimal pH range for Karl Fischer titration is between 5 and 8. Outside this range, the reaction rate can be adversely affected.
Why do we use methanol in KF?
Answer: Methanol is used in Karl Fischer titration because it dissolves the reagents and the sample well, and it reacts efficiently with sulfur dioxide and iodine.
Which solvent is used for KF?
Answer: Methanol is the most commonly used solvent in Karl Fischer titration. However, other alcohols like 2-(2-Ethoxyethoxy)ethanol (DEGEE) can also be used.
How to prepare KF solution?
Answer: KF solution is prepared by dissolving the necessary reagents (iodine, sulfur dioxide, and a base) in methanol or another suitable solvent. The exact composition depends on whether a one-component or two-component system is being used.
Why is water used in KF calibration?
Answer: Water is used in KF calibration to determine the titer of the Karl Fischer reagent, ensuring accurate and precise water content measurements.
What is KF analysis?
Answer: KF analysis refers to the process of measuring the water content in a sample using Karl Fischer titration.
Is KF dissolved in water?
Answer: No, Karl Fischer reagents are not dissolved in water. They are typically dissolved in methanol or other suitable solvents to facilitate the titration process.
How many gm iodine is used in KF reagent?
Answer: The amount of iodine in Karl Fischer reagent can vary, but typically, the reagent is formulated to contain about 1% to 5% iodine by weight.
What is K reagent?
Answer: K reagent, also known as Karl Fischer reagent, is a solution containing iodine, sulfur dioxide, and a base in an appropriate solvent, used for determining water content in samples.
Why is pyridine used in KF?
Answer: Pyridine was historically used as the base in Karl Fischer reagents to buffer the solution and stabilize the reaction intermediates.
Why is KF reagent pyridine free?
Answer: KF reagent is now pyridine-free because pyridine is a noxious and carcinogenic substance. Modern reagents use less hazardous bases like imidazole or primary amines.
How to regenerate a KF electrode?
Answer: To regenerate a KF electrode, it should be cleaned thoroughly with methanol or an appropriate solvent, then reconditioned by soaking in a KF reagent solution before use.


Yes, I with you definitely agree