Meaning of HVAC: Heating, Ventilation, and Air Conditioning:
Pharma formulation plants have a clean room, in which the drugs formulate into finished good products, and have a controlled atmosphere in these areas in terms of the controlled temperature, relative humidity, and particles. The Heating, Ventilation, and Air Conditioning are used to protect the Product, Personnel, and Environment. HVAC directly impacts product quality.
It can provide comfortable conditions for operators. This impacts the premises and prevention of contamination and cross-contamination to be considered at the design stage Temperature, and relative humidity control where appropriate.
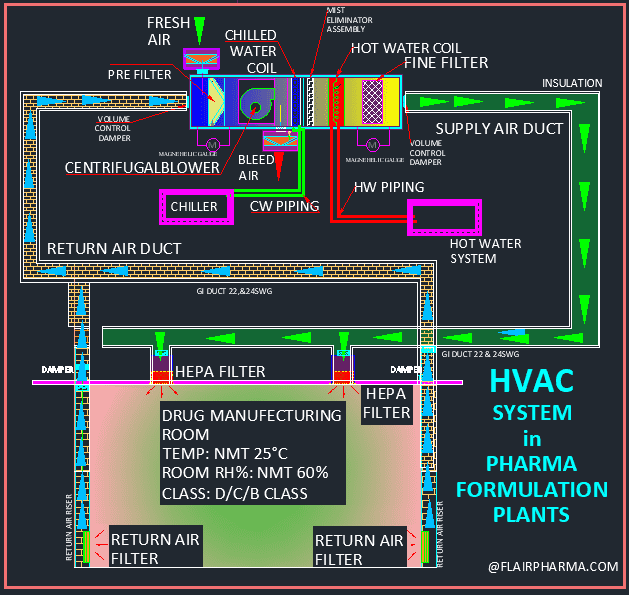
HVAC is a system with which Following parts:
- Air Handling Unit (AHU).
- Ducting and Piping.
- Air Filters (pre, Fine, and HEPA filters).
- Chilled water or Hot water Coils to control temperature and RH in the area.
- Control/ Monitoring Systems or BMS Integration.
Key Parameter of HVAC System:
- Temperature (Generally it’s NMT 25C).
- Relative humidity (e.g., NMT 60%).
- Air movement (Flow of air e.g., Unidirectional flow or turbulent flows).
- Microbial contamination.
- Particulate contamination (different area classification as per ISO).
Guidelines of different Authorities worldwide:
- ISO 14644.
- GHO(Annex-8).
- EU GMP Guidelines.
- PICS Guidelines.
Process of designing, Procurement, and installation of HVAC systems in Pharma:
- Preparation of RDS i.e. Room Datasheet.
- Design Basis Document (DBD).
- General Drawings and P&ID drawings preparation and approvals.
- Tender Document preparations.
- Technical bid Analysis (TBA).
- Purchase order finalization.
- Design Qualification review and its approval.
- Factory acceptance tests of AHUs,s and concerning equipment of the system.
- Installation of Air Handling Units, Ducting, Filters, Cleanroom monitors, etc.
- Installation Qualification.
- Operational Qualification.
- Performance Qualification.
4 basic categories of HVAC systems:
The Four Fundamental HVAC System categories are based on utilization. The following are the four primary types of HVAC systems:
-
- Split heating and cooling systems.
- split-system hybrids.
- Mini-split (without duct).
- Bundled systems (HVAC with AHU).
Followings different tests are performed to qualify the HVAC system as per ISO:
- Air velocity measurement.
- HEPA Filter installation leak test (DOP).
- Calculation of the Number of Air changes.
- Differential pressure crosses the cubicles.
- Temperature & RH measurement.
- Airborne particle counting.
- Power failure recovery study.
- Microbial monitoring.
Different Types of air handling Systems:
- Once through
- Re-circulation
- Ventilation
Once Through: The conditioned air, before it enters the room and then is discarded.
Advantages of a once-through system:
- Lots of fresh air is delivered by this system.
- This system can handle hazardous materials but must clean up air leaving the space.
- The exhaust duct is usually easy to route because of high velocity = smaller diameter.
Disadvantages:
- Expensive to run, especially when rooms required cooling and heating.
- Filter replacement is very frequent.
- Possibility of dust collection/scrubbers/cleanouts
Re-circulation: Air is conditioned before entering the room and a portion of it is reconditioned. Some of them may be discarded.
Advantages:
1. Less air filter loading usually means less filter maintenance and energy costs.
2. Possibility of improved air filtration because of recirculation.
3. Less of a challenge and better parameter control (Temp, RH, etc).
4. Less wasted air with lower cooling/heating losses.
Disadvantages:
1. The routing of return air ductwork to the air handler may be complicated above the ceiling.
2. The possibility of cross-contamination necessitates an adequate supply of air filtration (and occasionally return air filtration).
Ventilation: In this system, there is no system for treating the air, it’s a simple circulation of air from the atmosphere to the working area.
HVAC Achievements for clean area classification depend on the following factors:
- Building finishes and structure.
- Air filtration.
- Air change rate.
- Room pressure.
- Relative humidity.
- Material and personnel flow.
- Outside environment.
- Occupancy and type of product.
Do you know what is the need for this whole system i.e., HVAC, the answer is contamination and Cross contaminations in the atmospheric air that impacts the finished drug’s overall quality.
What is contamination?
It is “the undesired introduction of impurities (chemical/ microbial/ foreign matter into or on to starting material or intermediate – during sampling, production, packaging or repackaging”.
Impurities could include products or substances other than the product manufactured, foreign products, particulate matter, micro-organisms, endotoxins (degraded microorganisms), etc.
What are Cross-contaminations?
“Contamination of a starting material, intermediate product, or finished product with another starting material or product during production”.
The followings are the main reasons for Cross-contamination:
- Poorly designed, operated, or maintained air-handling systems and dust extraction systems.
- Improper working procedures, and movement of personnel, materials, and equipment.
- Less usable cleaned equipment as per capacities.
An uncontrolled environment can lead to product degradation.
- product contamination (including cross-contamination).
- Loss of product and profit.
To minimize Cross-contamination you have to review the following systems:
- Personnel procedures.
- Adequate premises.
- Utilization and practice of daily operation of the manufacturing area.
- Proper and approved validated cleaning process and procedures.
- Appropriate levels of protection of the product.
- Validated Air cascade Or pressure differential of two areas.
The worldwide guidelines further focus on three concepts of the system:
- Product protection.
- Contamination
- Cross-contamination
- Environmental conditions
- Personnel protection.
- Prevent contact
- Comfort conditions
- Environment protection.
- Protection: Product and personnel
- Areas, where materials and products are exposed, should be classified as “clean areas”
- Air filtration and air change rate should ensure attainment of classification Air change rate is dependent on factors, e.g.
- The level of protection required.
- Quality and filtration of supply air.
- Particulates generated.
- Room configuration.
- Containment effect.
- Room heat load.
- Room pressure.
- The air change rate normally varies between 20 – 120 air changes per hour depending on the class of that area.
The maximum permitted total particle concentration for classification:
| Grade | Maximum limits for total particles≥ 0.5 μm/m3 | Maximum limits for total particles≥ 5 μm/m3 | ||
| At Rest | In Operation | At Rest | In Operation | |
| A | 3 520 | 3 520 | Not specified (a) | Not specified (a) |
| B | 3 520 | 352 000 | Not specified (a) | 2 930 |
| C | 352 000 | 3 520 000 | 2 930 | 29 300 |
| D | 3 520 000 | Not predetermined (b) | 29 300 | Not predetermined (b) |
(a) Classification including 5μm particles may be considered where indicated by the CCS or historical trends.
(b) For grade D, operation limits are not predetermined. The manufacturer should establish operation limits based on a risk assessment and routine data where applicable.
Level of protection and air cleanliness determined according to:
- Product to be manufactured.
- The process to be used.
- Product susceptibility to degradation.
Parameters influencing Levels of Protection
- The number of particles in the air, and the number of microorganisms in the air or on surfaces.
- The number of air changes for each room.
- Air velocity and airflow pattern.
- Filters (type, position).
- Air pressure differentials between rooms.
- Temperature, relative humidity.
HVAC systems in manufacturing facilities are closely monitored by every regulatory authority like USFDA, EUGMP, MHRA, PiCS, etc. FDA and must adhere to other global current good manufacturing practices (cGMPs). For Sterile manufacturing, cleanrooms have many distinct characteristics:
- The air should have a high microbial quality.
- The air handling system includes a central HEPA filter bank as well as mandatory terminal filters.
- The filtration regime is typically three stages long, with two pre-filter stages of 10 microns, 3 microns, and one central final filter of 1 micron, as well as a terminal HEPA filter of 0.22 microns.
- All sterile critical operations must be performed under qualified laminar flow.
- Critical areas should have a positive pressure differential compared to adjacent less clean areas.
- Supply air outlets with perforated stainless steel grilles and terminal absolute filters are installed flush with the ceiling. Return air grilles with a return air riser will be installed at the floor level for improved scavenging.
- Clean areas’ walls, floors, and ceilings must be made of smooth, cleanable surfaces that are impervious to sanitizing solutions and resistant to chipping, flaking, and oxidizing.
- It is very critical to maintaining a proper pressurization gradient between adjacent spaces in order to prevent infiltration and cross-contamination.
- To improve the finished product and reduce energy consumption, air filtration techniques and air conditioning components must be constantly monitored and upgraded.
- HVAC capacity is required for higher air flows and pressures. Because the majority of engineering decisions will have an impact on HVAC systems, it is critical to identify opportunities to seek the best engineering solutions.
FAQ:-
What is the meaning of HVAC?
Answer: Heating, ventilation, and air conditioning are collectively known as HVAC. It is the devices that control a building’s temperature and ventilation, partial & contaminations
What is difference between AC and HVAC?
Answer: AC is used to control the temperature of the room only, But HVAC will be able to control temperature, relative humidity, particles, and airflow.
What is basic HVAC?
Answer: Heating, ventilation, and air conditioning are referred to as HVAC. The term HVAC refers to systems used for heating, cooling, and transporting air between the interior and outdoor spaces in pharmaceutical industries. HVAC is also used to control the contaminations and cross contaminations.
What are the three types of HVAC, AHU?
Answer: There are three types of AHU systems i.e. once through, Re-circulation type, and Ventilation type.
For more information, please write us at: admin@flairpharma.com

Good and informative
Great article on the importance of HVAC technology in the pharmaceutical industry! The information provided is very informative and helpful, especially for those who are not familiar with the industry. The article does a great job of highlighting the key features of HVAC systems used in pharmaceutical manufacturing, such as air filtration, temperature control, and humidity control. I also appreciate the emphasis on the importance of maintaining HVAC systems to ensure they function properly and meet regulatory requirements. Overall, this article is a valuable resource for anyone looking to learn more about the role of HVAC technology in the pharmaceutical industry. Keep up the good work!