Technology transfer in the pharmaceutical industry is a critical step in bringing a new drug or medical device from the laboratory to the market. It involves transferring knowledge, expertise, and technology from the research and development phase to manufacturing, quality control, and regulatory compliance. Technology transfer is essential to the process of discovering novel drugs and developing new pharmaceuticals. The choice to move production between facilities is frequently influenced by economics. Data gathering, data evaluation, regulatory impact, with a focus on any modification approvals, analytical validation, pilot or full-scale process batch, and stability set down are important steps of the process (if required).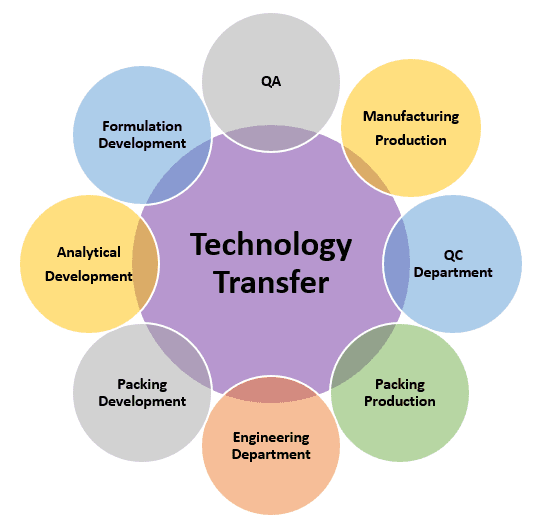
The following is an overview of the typical steps involved in the technology transfer process in the pharmaceutical industry:
- Feasibility assessment: The first step in technology transfer is to evaluate the feasibility of the process. This involves assessing the technology readiness level, potential risks and benefits, and regulatory requirements.
- Process development: Once feasibility is established, the next step is to develop the process for manufacturing the product. This includes optimizing the production process, establishing quality control measures, and identifying raw materials and equipment requirements.
- Transfer plan: A detailed transfer plan is developed to document the entire technology transfer process, including timelines, responsibilities, and quality standards.
- Manufacturing: The manufacturing process is then validated to ensure that the product is consistently produced to meet the required quality standards.
- Regulatory compliance: The product must comply with all applicable regulatory requirements, including those related to safety, efficacy, and labeling. The transfer team works closely with regulatory authorities to ensure compliance.
- Technology transfer completion: Once the manufacturing process is established, the technology transfer process is considered complete. The product can then be commercialized and marketed to consumers.
The technology transfer process in the pharmaceutical industry is complex and involves multiple stakeholders, including scientists, engineers, manufacturing personnel, quality assurance professionals, and regulatory authorities. Effective communication and collaboration are essential to ensure a successful transfer of knowledge and technology from research and development to commercialization.
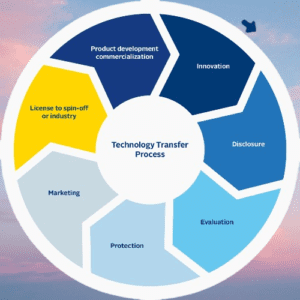
Major points impacted during Technology transfer:
- Scale and batch size adjustments.
- Modifications to the production route.
- Raw material modifications.
- Equipment alterations.
- Changes to the production location.
The final medication product’s Critical Quality Attributes (CQAs) might be significantly impacted by any alteration. Changes that routinely fail to meet CQA requirements must be avoided for obvious reasons.
Key Factors of Tech Transfer
Technology transfer is the process of transferring technology from one organization to another, and it plays a crucial role in the pharmaceutical industry. This process enables companies to develop and produce new products, improve existing ones, and increase their competitiveness in the market. The following are the key factors in technology transfer in the pharmaceutical industry:
- Communication: Effective communication between the sending and receiving units is crucial to the success of technology transfer. It is essential to establish clear and open channels of communication, share information about the technology, and clarify expectations.
- Quality Management System: A well-defined Quality Management System (QMS) is necessary to ensure that the technology transfer process is performed according to established procedures and meets the requirements of all relevant regulatory agencies. The QMS should include procedures for documentation, training, change control, and risk management.
- Knowledge Management: Knowledge transfer is a key component of technology transfer. It involves transferring knowledge and expertise from the sending unit to the receiving unit. This includes knowledge of the product, process, equipment, and analytical methods. Knowledge management systems such as databases, SOPs, and training programs should be established to facilitate knowledge transfer.
- Technical Capability: The receiving unit must have the technical capability to receive and implement the technology. This includes having the appropriate facilities, equipment, and personnel with the necessary technical expertise.
- Regulatory Compliance: Compliance with regulatory requirements is critical in the pharmaceutical industry. The receiving unit must be able to meet all regulatory requirements for the product, process, and facility.
- Risk Management: Risk management is an essential component of technology transfer. Risks should be identified, assessed, and mitigated to ensure that the process is performed safely and efficiently. If anything let’s cover this with deviations.
- Project Management: Project management is crucial to the success of technology transfer. It involves establishing timelines, milestones, and budgets, and monitoring progress to ensure that the project is completed on time and within budget.
In conclusion, technology transfer is a complex process that requires a multidisciplinary approach. Effective communication, a well-defined quality management system, knowledge management, technical capability, regulatory compliance, risk management, and project management are key factors that contribute to the success of technology transfer in the pharmaceutical industry.
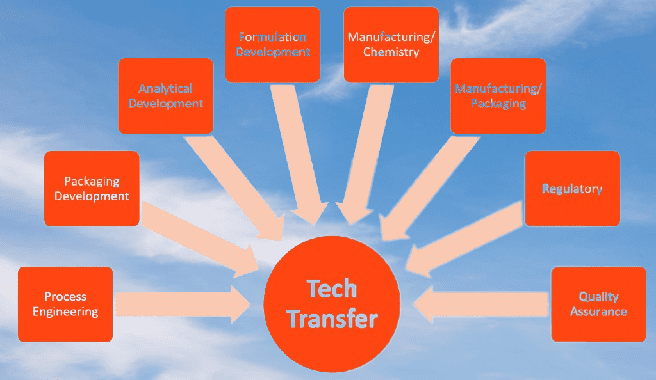
The technology transfer process
The tech transfer process in the pharmaceutical industry involves the transfer of knowledge, technology, and expertise from one organization to another. It typically involves the transfer of a product, process, or analytical method from the development or manufacturing site to the commercial manufacturing site.
The following is a general outline of the technology transfer process in the pharmaceutical industry:
- Planning: The technology transfer process typically begins with planning. This includes identifying the product or process to be transferred, establishing timelines, and identifying the sending and receiving units.
- Documentation: The documentation process includes generating and reviewing technical documents, such as master batch records, standard operating procedures (SOPs), and protocols. These documents are critical to ensuring that the technology transfer process is consistent and repeatable.
- Training: The training process involves ensuring that personnel involved in the technology transfer process are trained on the product, process, and equipment. This includes training on SOPs, batch records, and analytical methods.
- Transfer Verification: Transfer verification involves confirming that the product or process has been successfully transferred from the sending site to the receiving site. This includes verifying that the product meets all specifications and that the process is capable of producing the product consistently.
- Commercialization: The final step in the technology transfer process is commercialization. This involves scaling up the process to full commercial production and obtaining regulatory approval to market the product.
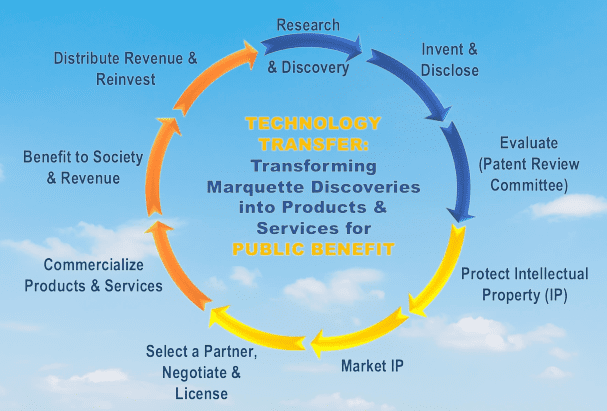
Throughout the technology transfer process, it is essential to maintain effective communication between the sending and receiving units. This includes regular meetings, sharing of technical data and documentation, and addressing any issues or concerns that may arise.
It is also critical to maintaining a well-defined Quality Management System (QMS) throughout the technology transfer process. This includes procedures for documentation, training, change control, and risk management to ensure that the process is performed according to established procedures and meets the requirements of all relevant regulatory agencies.
In conclusion, the technology transfer process in the pharmaceutical industry is a complex process that requires a multidisciplinary approach. Effective planning, documentation, training, transfer verification, and commercialization are essential steps in the process, along with maintaining effective communication and a well-defined QMS.
