Deionized (DI) water is a form of purified water with most or all of its mineral ions removed, such as cations like sodium, calcium, iron, and copper, and anions like chloride and sulfate. The pH level of deionized water is a critical parameter, especially in high-purity applications, but it presents unique challenges due to its nature. This article explores the relationship between pH and deionized water, elaborating on the complexities of measuring pH in such pure water.
Understanding pH
The pH scale, ranging from 0 to 14, measures the hydrogen ion concentration in a solution. A pH value of 7 is considered neutral, values less than 7 are acidic, and those greater than 7 are basic. The pH scale is logarithmic, meaning a small change in pH represents a significant change in hydrogen ion concentration.

Measuring pH in Deionized Water
Measuring the pH of deionized water is notoriously difficult due to its very low ionic strength. This low ionic strength causes several issues:
- Unstable Readings: The lack of ions makes it hard for pH electrodes to achieve the required electron transport between the measuring and reference sides, leading to unreliable readings.
- Contamination Sensitivity: Deionized water can quickly absorb carbon dioxide from the air, forming carbonic acid and lowering the pH. This means that even brief exposure to air can significantly alter the pH reading.
- Buffering Issues: DI water has little to no buffering capacity, making it susceptible to pH changes upon minimal contaminant introduction.
These factors contribute to the challenge of obtaining accurate pH measurements using standard pH meters, which are often calibrated for solutions with higher ionic strengths.
Alternatives to Direct pH Measurement
Given the difficulties with direct pH measurement, alternative methods are often employed:
- Inline pH Meters: These devices avoid atmospheric contamination and use special high-resistivity electrodes with temperature compensation, providing more accurate readings. However, they are not foolproof and can still suffer from contamination by the chemicals in the reference electrodes.
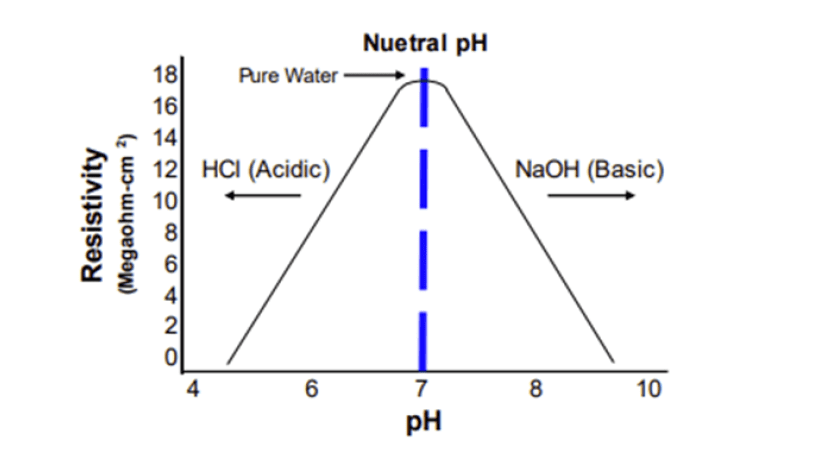
- Resistivity Meters: A practical alternative is using a resistivity meter to infer the pH of deionized water. Pure water with a resistivity of 18.2 Megaohms (or conductivity of 0.055 µS) is generally considered to have a neutral pH. As resistivity decreases, indicating increased ionic presence, the potential pH range broadens. The specific pH value depends on the types of salts present in the water.
The Role of Salts
The type of salts present in the water influences the pH significantly:
- Strong Acid + Strong Base: Produces a neutral solution.
- Strong Acid + Weak Base: Results in an acidic solution.
- Weak Acid + Strong Base: Leads to a basic solution.
- Weak Acid + Weak Base: Can produce an acidic, neutral, or basic solution, depending on the specific salts involved.
The table below summarizes the relationship between resistivity and the potential pH range:
| Resistivity (Megaohms) | Conductivity (µS) | Max pH | Min pH |
| 18.2 | 0.055 | 7 | 7 |
| 18 | 0.056 | 7.8 | 6.2 |
| 16 | 0.063 | 7.9 | 6.1 |
| 10 | 0.1 | 8.1 | 5.9 |
| 5 | 0.2 | 8.4 | 6.5 |
| 2 | 0.5 | 8.8 | 5.2 |
Practical Implications
For practical purposes, obtaining a neutral or near-neutral pH in deionized water can be more reliably achieved by using resistivity measurements rather than pH meters. Inline resistivity meters provide a robust solution for monitoring high-purity water systems, minimizing contamination risks.
In applications requiring specific pH ranges, understanding the types of salts present and their interactions with water is crucial. The variability in pH based on resistivity highlights the need for careful control and monitoring in high-purity water systems.
Conclusion
The relationship between pH and deionized water is complex, primarily due to the challenges in measuring pH accurately in such a low ionic strength environment. By leveraging resistivity measurements and understanding the chemistry of salts in water, more reliable inferences about pH can be made. This approach helps in designing and troubleshooting high-purity water systems effectively.
Frequently Asked Questions (FAQ’s)
How does deionized water affect pH?
Answer: Deionized water can have an unstable pH because it lacks ions and has little buffering capacity. This means its pH can be easily influenced by even small amounts of contaminants or exposure to air, which can introduce carbon dioxide and form carbonic acid, lowering the pH.
Will DI water change the pH?
Answer: Yes, deionized water can change pH easily upon exposure to air or contaminants. Its pH is not stable due to the absence of buffering ions, so it can absorb carbon dioxide from the air, forming carbonic acid and lowering the pH.
What is the pH and conductivity of DI water?
Answer: The pH of pure deionized water is ideally around 7 (neutral), but in reality, it can be slightly acidic (around 5.6) due to carbon dioxide absorption from the air. The conductivity of ultra-pure deionized water is 0.055 µS/cm, corresponding to a resistivity of 18.2 Megaohms.
What is the pH of RO DI water?
Answer: The pH of reverse osmosis (RO) deionized water is typically close to neutral (around 7) but can be slightly acidic due to the same reasons affecting regular deionized water, such as carbon dioxide absorption.
What is the pH of deionized water with NaOH?
Answer: Adding NaOH (a strong base) to deionized water will increase its pH significantly. The exact pH depends on the concentration of NaOH added, but it will generally be in the basic range (above 7).
What is TDS of distilled water?
Answer: The Total Dissolved Solids (TDS) of distilled water is very low, typically less than 2 mg/L, as most dissolved ions are removed during the distillation process.
What is the pH of boiled deionized water?
Answer: Boiling deionized water can drive off dissolved gases like carbon dioxide, potentially raising its pH closer to 7. However, the exact pH after boiling can vary depending on contamination and handling.
What are the disadvantages of deionized water?
Answer:
- Unstable pH
- Highly reactive and can corrode certain materials
- Lacks essential minerals, making it unsuitable for drinking
- Can quickly absorb contaminants from the environment
How to calculate pH?
Answer: The pH is calculated using the formula:

What is the pH of pure water?
Answer: The pH of pure water at 25°C (77°F) is 7, which is considered neutral.
What is the pH of NaOH?
Answer: The pH of a sodium hydroxide (NaOH) solution depends on its concentration. For example, a 0.1 M NaOH solution has a pH of about 13.
What is normal pH in RO?
Answer: The normal pH of water produced by reverse osmosis systems is typically close to neutral, around 7, but it can be slightly acidic (between 5 and 7) due to the removal of buffering minerals.
Is RO or DI water more pure?
Answer: Deionized water is generally more pure than RO water because deionization removes both organic and inorganic ions, while RO primarily removes dissolved salts and larger molecules.
How to raise pH of RO water?
Answer: The pH of RO water can be raised by adding alkaline minerals such as calcium or magnesium or by using a post-treatment alkaline filter.
What is the pH for deionised water?
Answer: The pH of deionized water is ideally 7, but it can range from 5.6 to 7 due to carbon dioxide absorption and lack of buffering capacity.
What is meant by deionized water?
Answer: Deionized water is water that has had most or all of its mineral ions removed, including cations like sodium and calcium and anions like chloride and sulfate, usually through ion exchange processes.
What is the difference between distilled and deionized water?
Answer:
- Distilled water is produced by boiling water and condensing the steam, removing impurities and minerals.
- Deionized water is produced by passing water through ion exchange resins, removing dissolved salts and ions.
What’s the formula for deionized water?
Answer: The chemical formula for deionized water is the same as regular water, H2O\text{H}_2\text{O}H2O.
Can you drink deionized water?
Answer: While it is technically safe to drink deionized water, it is not recommended because it lacks essential minerals and can taste flat or unpleasant. It can also leach minerals from the body over time.
What are the properties of deionized water?
Answer:
- Very low ionic content
- High resistivity (up to 18.2 Megaohms)
- Unstable pH
- High reactivity and solvent capacity
- Often used in industrial processes and laboratory applications where pure water is required
