In the complex and highly regulated pharmaceutical industry, QMS helps to delivering products of the highest quality and safety is non-negotiable. This responsibility is anchored in the implementation of a robust Quality Management System (QMS). A QMS serves as a strategic framework that ensures the consistent delivery of resources, products, and services, meeting the stringent quality and safety standards expected by healthcare professionals, patients, and regulatory authorities.
QMS: A Systematic Framework
At its core, a QMS is a set of interacting elements—procedures, policies, resources, and objectives—designed to guide an organization toward quality excellence. It integrates regulatory guidelines and organizational policies to maintain robustness, adaptability, and effectiveness.
For pharmaceutical companies, a QMS is indispensable. It bridges the gap between product development and manufacturing, ensuring that processes are streamlined, deviations minimized, and risks managed proactively.
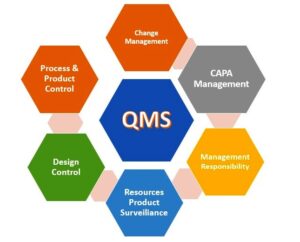
Components of a Pharmaceutical QMS:

The effectiveness of a QMS in the pharmaceutical sector hinges on several critical components:
Impact of QMS on the Pharmaceutical Industry:
The pharmaceutical landscape has evolved significantly, driven by changing market demands and regulatory expectations. A QMS provides the tools to navigate these shifts effectively:
- Enhancing Product Quality
By aligning with international standards, a QMS ensures medicines consistently meet the needs of healthcare providers and patients. - Strengthening Compliance
Regulatory bodies demand strict adherence to guidelines, and a QMS ensures compliance while facilitating innovation and flexibility. - Fostering Efficiency
Automation and standardization within a QMS streamline operations, reduce variability, and optimize resources. - Driving Innovation
A well-implemented QMS supports research and development by transferring knowledge seamlessly from the lab to manufacturing, enhancing the scalability and efficacy of new drugs.
Implementation of QMS in the Pharmaceutical Sector:
The successful implementation of a QMS involves several critical steps, systematically planned and documented to ensure consistency and transparency:

Quality Management System (QMS): The Backbone of Excellence
A Quality Management System (QMS) serves as the backbone of any organization’s drive for excellence. More than a set of guidelines, QMS is a philosophy, a framework, and a culture that ensures products and services consistently meet or exceed customer expectations. Let’s delve into the intricacies of QMS in a unique way by exploring its core components, benefits, challenges, and evolution—woven together as an analogy to a well-orchestrated symphony.
The Symphony of Quality: A Harmonious Framework
Imagine QMS as a grand symphony orchestra. Each section—strings, brass, woodwinds, and percussion—represents a crucial aspect of quality management. When each section performs its role flawlessly, the result is a melody of success that resonates with stakeholders.
- The Strings: Policies and Objectives
The strings set the tone, much like QMS policies establish the foundation. These policies outline the organization’s commitment to quality, its vision, and measurable objectives. - The Woodwinds: Processes and Procedures
Woodwinds add nuance, just as well-defined processes and procedures provide clarity. Every department contributes its unique “note,” ensuring consistency and smooth workflows. - The Brass: Audits and Reviews
The brass section ensures robustness, akin to internal audits and management reviews. These mechanisms check for alignment with quality standards and identify areas needing fine-tuning. - The Percussion: Corrective and Preventive Actions
Percussion delivers precision and rhythm, mirroring corrective and preventive actions. These actions address non-conformities and proactively mitigate risks to ensure continuous improvement.
Why Every Organization Needs a QMS:
A QMS isn’t just a luxury for large corporations; it’s a necessity for any entity striving for growth, customer satisfaction, and sustainability.
- Consistency in Deliverables
A QMS ensures that processes are replicable, reducing variability in products and services. This consistency builds trust among customers and stakeholders. - Regulatory Compliance
Industries like healthcare, aerospace, and food production have stringent regulatory requirements. A QMS ensures compliance, avoiding legal pitfalls and maintaining brand integrity. - Enhanced Customer Satisfaction
By focusing on customer requirements and feedback, a QMS fosters loyalty and improves the overall customer experience. - Operational Efficiency
Standardized processes reduce waste, streamline operations, and optimize resource utilization.
The Challenges in Implementing a QMS
Even the most harmonious symphony can encounter discord. Implementing and maintaining a QMS comes with its challenges:
- Resistance to Change
Employees accustomed to existing workflows may resist the shift to a more structured system. - Resource Constraints
Small and medium-sized enterprises (SMEs) often struggle with the financial and human resources needed for QMS implementation. - Over-complexity
Over-engineering a QMS can lead to confusion and inefficiencies. Simplicity and scalability are key. - Continuous Maintenance
A QMS is not a “set-it-and-forget-it” system. Regular audits, updates, and employee training are essential for sustained success.
Future Directions and Challenges
The QMS in the pharmaceutical industry continues to evolve with advancements in technology and increasing regulatory scrutiny. The integration of digital tools such as predictive analytics, IoT, and AI is transforming quality management, offering real-time monitoring and predictive capabilities.
However, challenges remain. Resistance to change, resource constraints, and the complexity of regulatory environments demand a tailored approach to QMS implementation, particularly for small and medium-sized enterprises.
Conclusion
A Quality Management System is more than a compliance tool; it is a cornerstone of operational excellence and innovation in the pharmaceutical industry. By fostering a culture of quality, ensuring regulatory adherence, and enabling continuous improvement, a QMS not only supports public health but also empowers organizations to thrive in a competitive market.
In a world where the stakes are high, and lives depend on the integrity of medicines, the QMS serves as the guiding star, ensuring that every product delivered meets the highest standards of quality, safety, and reliability.
Frequently asked questions (FAQ):
What is in a quality management system?
A Quality Management System encompasses policies, procedures, processes, and resources that align organizational goals with quality assurance. It includes:
- Documentation: Manuals, SOPs, and records.
- Processes: Workflow standardization.
- Control Measures: Risk management, audits, and feedback mechanisms.
- Continuous Improvement: Methods like PDCA (Plan-Do-Check-Act).
What are the 7 principles of QMS?
The seven principles are:
- Customer Focus: Delivering value consistently.
- Leadership: Guiding teams towards a unified goal.
- Engagement of People: Harnessing collective skills and experience.
- Process Approach: Streamlining interconnected activities.
- Improvement: Driving innovation and addressing inefficiencies.
- Evidence-Based Decision Making: Relying on accurate data.
- Relationship Management: Fostering collaboration with stakeholders.
What are the 4 types of quality management?
- Total Quality Management (TQM): Holistic, company-wide quality culture.
- Six Sigma: Data-driven approach to reduce defects.
- Lean Management: Focus on waste elimination.
- ISO-Based Systems: Adhering to standardized frameworks like ISO 9001.
What are the 7 QC tools?
- Cause-and-Effect Diagram (Ishikawa).
- Control Charts.
- Flowcharts.
- Pareto Analysis.
- Histogram.
- Scatter Diagram.
- Check Sheets.
Why is a QMS important?
A QMS ensures consistent quality, regulatory compliance, customer satisfaction, and operational efficiency. It also drives innovation and fosters a culture of accountability.
What is QMS format?
The QMS format typically includes a Quality Manual, Standard Operating Procedures (SOPs), records, and templates for documenting compliance and improvements.
What is QMS checklist?
A QMS checklist is a structured tool used during audits to ensure all quality management requirements, such as ISO 9001 clauses, are met systematically.
What do QA and QC stand for?
- QA (Quality Assurance): Proactive processes to prevent defects.
- QC (Quality Control): Reactive measures to detect and correct defects.
What is quality in ISO?
Quality in ISO refers to meeting or exceeding stakeholder expectations through consistent processes, as outlined in standards like ISO 9001.
What are the 7 steps of QMS?
- Define Quality Objectives.
- Develop Processes.
- Document Procedures.
- Implement Controls.
- Train Teams.
- Monitor and Measure.
- Drive Continuous Improvement.
What is risk-based thinking?
Risk-based thinking emphasizes identifying, evaluating, and mitigating risks proactively in decision-making and process planning.
Who needs a QMS?
Any organization that values consistent quality, compliance, and customer satisfaction—from manufacturing to healthcare to IT services—needs a QMS.
What is checklist in TQM?
In Total Quality Management (TQM), a checklist ensures that all activities align with quality goals, from production to service delivery.
What is QMS testing?
QMS testing validates the effectiveness of a system in meeting predefined quality objectives through audits, simulations, and inspections.
What are the 4 types of QMS?
- ISO 9001: General quality standards.
- ISO 13485: For medical devices.
- GMP (Good Manufacturing Practices): For pharmaceuticals.
- HACCP: For food safety.
What are QC documents?
Documents that track quality control activities, such as test reports, inspection records, and corrective action reports.
What are the 9 quality standards?
Examples include:
- ISO 9001
- ISO 13485
- ISO 14001
- ISO/TS 16949
- ISO 22000
- ISO 45001
- ISO 50001
- GMP
- Six Sigma standards.
How to write a QMS?
- Define the scope.
- Document policies and objectives.
- Develop SOPs.
- Include risk assessment and control measures.
- Set up monitoring and review mechanisms.
What is a quality manager?
A quality manager oversees QMS implementation, ensuring compliance, managing audits, and fostering a culture of continuous improvement.
What are the six quality systems?
- Change Control.
- Deviation Management.
- CAPA.
- Document Control.
- Training and Qualification.
- Audits and Inspections.
Who is the father of 7 QC tools?
Dr. Kaoru Ishikawa is credited as the father of the 7 QC tools.
What is Six Sigma in quality management?
A methodology that reduces defects by improving process capabilities, aiming for near-perfection with 3.4 defects per million opportunities.
What is ISO certification?
An official recognition that an organization adheres to internationally recognized standards, like ISO 9001 for quality management.
What is the TQM model?
The Total Quality Management model emphasizes customer focus, continuous improvement, and team involvement to achieve quality excellence.

1 thought on “Quality Management System (QMS)”