Manufacturing Process of Oral Solid Dose (Tablet/Capsule):Oral Solid doses are the pharmaceutical dosage forms i.e., Tablets/capsules containing drug substances with or without suitable diluents and prepared by compression or molding manufacturing process methods. Oral Solid Doses (Tablet/capsule) have a huge market all around the world market. Oral solid doses are the manufacturing process comprising a mixture of (API) active substances and excipients, generally in the form of powder & pressed into the form of a tablet or fill it into capsules and form a solid dose. Manufacturing processes are as per the cGMP and GMP standards. Patients all over the world understand and accept tablets and capsules.
Index:
- Types of Tablets.
- Types of capsules.
- Process Flow Diagram.
- The test involved in QC Lab.
- Major Documentation.
- Critical process parameters.
- Conclusion.
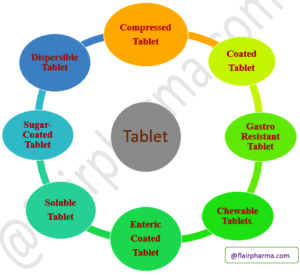
Type of tablets in pharmaceuticals and their manufacturing Process:
- Uncoated or compressed tablet.

Types of Tablets - Coated tablet.
- Chewable Tablets.
- Effervescent Tablets.
- Sugar-coated Tablets.
- Gastro-resistant tablet.
- Dispersible Tablet.
- Enteric-coated Tablet.
- Soluble Tablet.
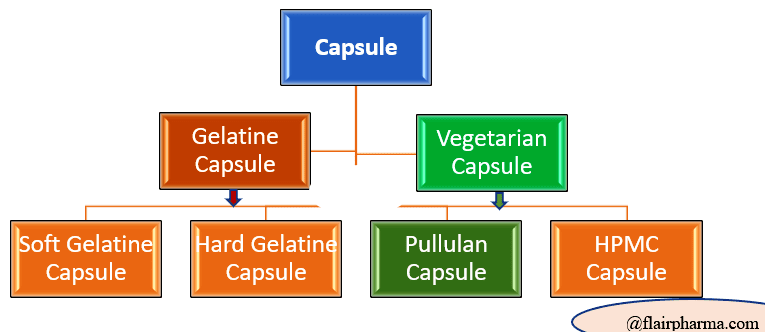
Types of capsules in pharmaceuticals:
- Hard Gelatine Capsule

Types of Capsules - Soft Gelatine Capsule
- HPMC Capsule
- Pullulan Capsule
- Enteric Coated Capsule
Process Flow Diagram of Manufacturing Process of Oral Solid Dose (Tablet/Capsule):
- Dry Granulation by RMG, FBD, Sifter, and Blenders.
- Wet Granulation: Suitable for drugs that are stable to moisture and heat.
- Direct Compression: Sensitive to moisture and heat. (by Compression Machine)
- Compression by tablet compression machine.
- Film/Suger Coating by tablet coating machine.
- Packing by Strip Packaging, Blister packaging, or Bottle packing machine.
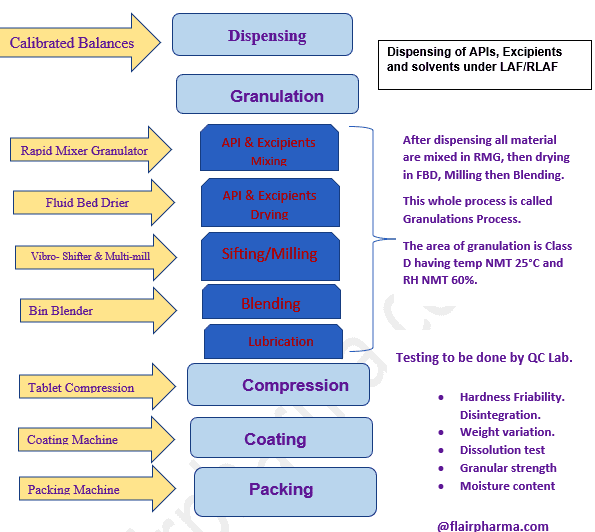
Test Involve in Quality control Lab for Tablet/Capsule Manufacturing Process:
- Hardness Friability Disintegration
- Weight variation
- Dissolution test
- Granular strength
- Moisture content
Major Documentation for Tablet/Capsule manufacturing process.
- MFR – Master formula record.
- BMR – Batch manufacturing record.
- BPR – Batch packing record
- COA – Certificate of analysis.
Oral solid dosage forms (typically tablets and capsules) are made using traditional manufacturing techniques such as direct blending or granulation techniques such as Fluid bed granulation followed by fluid bed drying, milling, blending, compression, and coating.
The critical processing parameters (CPPs) play a very important role to impact critical quality attributes (CQA). For OSD particle size, morphology, dosage strength, and drug loading all have a significant impact on the blending process and can affect the homogeneity of the drug substance in the blend.
Blending is a critical process that must be optimized, and it is classified as a CPP because it can affect the potency of the finished product. Similarly, each of the granulation techniques has a processing step that can cause variability and affect the CQAs.
The API properties, stability, dose, drug loading, and other factors will all influence the key parameters that must be optimized.
Following are some process parameters while manufacturing Oral Solid Doses.
Process: Choose the process which suits best your product as per R&D and F&D.
Critical process parameters
Blending: Choose the type of blender
- Blending time
- Number of revolutions of the blender
- Rapid Mixing of API & excipients by RMG.
- High shear wet granulation
- Kneading time
- Impeller and chopper speed
- Binder addition time
- Fluid bed drying
- Inlet air temperature
- Fluidization air volume
- Dew point
- Product temperature
- Roller compaction
- Roll gap
- Roll width
- Roll pressure
- Screen size
- Fluid bed granulation
- Spray volume
- Spray rate
- Inlet air temperature
- Milling
- Screen size
- Compression
- Compression force
- Compression speed
- Dwell time
- Coating
- Spray rate
- Inlet air temperature
- Encapsulation
- Speed of encapsulation
- Tamping pressure
- Packaging
- Strip pack
- Blister pack
- Bottle pack
Conclusion:
the tablet and capsules can be formulated to deliver an accurate dosage to a specific form. OSD is always intake orally, but can be administered sublingually, buccally, rectally, or intravaginally.
The tablet and capsules are just one of the many forms that an oral drug can take such as syrups, elixirs, suspensions, and emulsions.
Tablets and capsules are often stamped with symbols, letters, and numbers, which enable them to be identified. Also, all testing is to be done by QC & microbiology lab by best laboratory practices.
For more details, please contact at:admin@flairpharma.com