The Pharmaceutical industries are the most promising sector of the Indian industry, which is growing at more than 9% per annum. So there are lots of opportunities in the pharmaceutical sector in India as well as the entire world. So, this post will assist you in understanding the fundamental structure for establishing new facilities and flying with pharmaceutical industrial growth.
Worldwide recognized quality standards : ∝
Pharma projects drive as per world markets & their regulatory authorities:
- WHO GMP norms.
- The more stringent GMP norms are being followed by highly regulated markets in developed countries like the USA (USFDA), Europe (EMEA), European Union GMP, UK (MHRA), Australia (TGA), South Africa (MCC), and Japan, etc.
- All developed countries have mostly common cGMP regulations like ICH i.e. International Conference on Harmonization (ICH) and many other different guidelines.
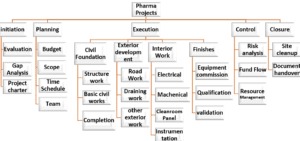
Projects Process Flow:
Pharma Projects⇓
1. initiation 2. Planning 3. Execution 4. Control 5. Closure
1.0 Initiation
1.1 Evaluation
1.2 Gap Analysis
1.3 Project charter
2. Planning
2.1 Budget
2.3 Scope
2.4 Time Schedule
2.5 Team
3.0 Execution
- Civil Foundation
- Exterior development
- Interior Work
- HVAC
- Electrical
- Equipment installations
- Electromechanical completion
4.0 Control
- Risk analysis
- Fund Flow
- Resource Management
5.0 Closure
- Site cleanup
- Document handover
- Audits
Project Process:
- Conceptual Design & Execution (Inhouse/Design Consultancy)
- Basic & Detailed Engineering
- Project Management Consultancy
- Procurement
- Support from all stakeholders
- Commissioning, Validation & Documentation
Detailed Engineering (Out Source):
- Eqpt. Design / Technical Spec.
- P&ID
- Automation & Networking.
- Clean room & HVAC Design.
- Electrical Engineering.
- Clean & Black Utility generation & Distribution.
- Piping Engineering.
- 2D, 3D Auto cad / Isometric drawings.
- Mass & Energy balance.
Conceptual Design:
- Facility Conceptual Design
- Process Flow Diagram
- URS Of Facility & equipment
- BOD (Basis of design )
- Site Master plan
- Clean room Development and concept
- Utility Estimation & Matrix
- Electrical Load Estimation
- Control Philosophy
- Tentative Project Cost Estimation
Project Management:
- Progress Chart with major milestone update.
- All stakeholder progress, requirements & coordination.
- DQ (Design Qualification), IQ (installation Qualification) & Protocol
- VMP
- Preparation and execution of OQ & PQ.
- Handover & Audits
Scope Management:
- This is a component of project management in the pharmaceutical sector.
- Planning, carrying out, and monitoring a project.
- Planning and managing a timeline and a budget.
- Management of stakeholders.
- Management of compliance and regulatory strategies.
- Environment protection.
- Risk control.
- Team leadership.
- This is a component of project management in the pharmaceutical sector.
For more details, please contact: admin@flairpharma.com
