When you think about Sustainable and Smart Cleanroom Strategies for Next-Gen Pharmaceutical Facilities what should come in your mind Cleanrooms or smart cleanrooms. Cleanrooms exist for a single, non-negotiable reason: to protect patients by controlling contamination risk. That purpose has not changed. What has changed is the context in which cleanrooms must now deliver.
In pharmaceuticals, the cleanroom is more than a controlled space—it is the final physical barrier between a patient and contamination.
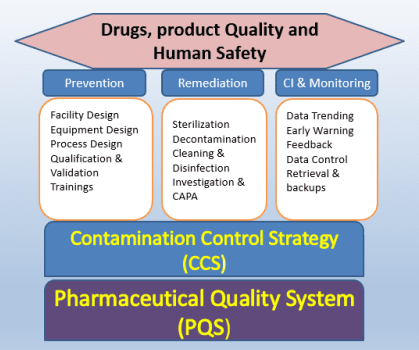
Across India and globally, cleanrooms are now expected to perform under pressures that were far less visible a decade ago: energy volatility, water stress, carbon accountability, fragile supply chains, and a shrinking pool of skilled operations and maintenance talent. At the same time, regulatory expectations are evolving. Compliance is no longer demonstrated only through periodic qualification; regulators now expect lifecycle evidence of control, anchored in a robust Contamination Control Strategy (CCS).
The outcome is clear. Future pharmaceutical cleanrooms must remain fully GMP-compliant while becoming significantly lighter on energy, water, and total lifecycle cost.
From the operations chair, sustainability is not viewed as an optional initiative or a branding exercise. It is a plant-reliability requirement. An oversized, wasteful system is inherently harder to stabilize, more likely to drift out of control, and more prone to deviations. In contrast, a cleanroom that is right-sized by risk and maintained within limits through continuous data verification is easier to operate, easier to defend during audits, and far more resilient over time.
This article outlines practical strategies to design and operate smart, sustainable cleanrooms. It also reflects common industry transformations that Indian pharmaceutical facilities can realistically adopt without compromising sterility assurance.
What Do We Mean by “Smart” and “Sustainable”?
A next-generation cleanroom is not defined by higher background grades or newer equipment alone. It is defined by the intelligence of its risk-to-resource alignment.
Smart Cleanrooms:
A smart cleanroom demonstrates four essential operational qualities:
- Measured
Critical environmental parameters are monitored using reliable, fit-for-purpose sensors. - Connected
Building Management Systems (BMS), Environmental Monitoring Systems (EMS), utilities, alarms, and maintenance platforms operate on a single source of truth. - Responsive
Control is dynamic rather than static. Airflow and utilities respond to actual occupancy, process state, and operational need. - Continuously Verified
Cleanroom health is demonstrated through daily trend data, not only during qualification or requalification windows.

Sustainable Cleanrooms:
A sustainable cleanroom is designed and operated so that:
- Air, energy, and water are consumed only to the extent that contamination risk genuinely demands.
- Lifecycle emissions, maintenance burden, and operational complexity are minimized at the design stage, not managed reactively after commissioning.
- Waste from consumables and maintenance is reduced without affecting contamination control.
The sharpest facilities treat smart capability as the method and sustainability as the result. Smart systems without sustainability become expensive toys. Sustainability without smart verification becomes fragile under audit pressure. - Why Cleanrooms Must Evolve Now:
1) Utilities Dominate the Site Footprint :
In sterile, biological, and high-containment facilities, HVAC is often the largest energy consumer. Within HVAC, air movement is the primary cost driver. Every additional ACPH increases fan power continuously—this is not a one-time CAPEX choice, but a 10–15 year OPEX and carbon commitment.
2) CCS Pushes Risk-Based Performance:
Modern regulatory expectations treat CCS as a living framework, discouraging blind inheritance of historical URS values and promoting performance-based, risk-justified control.
3) Resilience Is Now a Compliance Factor
Power variability, utility interruptions, and manpower scarcity are operational realities. Oversized systems with complex control loops are harder to stabilize and more deviation-prone. Efficiency and controllability now directly support compliance.
Strategy 1: Right Grade, Right Zone, Right Air
The strongest sustainability lever is also a GMP strength: avoid habitual over-grading and over-ventilation.
A. Address Grade Inflation
Long-running plants often carry legacy excess—corridors upgraded “just in case,” change rooms ventilated like compounding areas, or support rooms retaining critical specs long after processes moved. The result: high energy demand, unstable cascades, and frequent EMS alarms.
B. Zone by CCS Risk Questions
Grade selection must follow risk, not tradition:
Product risk – sterile vs non-sterile, potent vs conventional
Process risk – exposure duration, open handling, manual intensity
Environmental risk – personnel flow, gowning, occupancy density
Honest answers lead to defensible grades and rational resource use.
C. Replace Fixed ACPH with Validated Performance Bands
ACPH is a means, not the outcome. Outcomes are particle control, recovery time, and airflow stability. Best practice validates an ACPH operating envelope using:
• steady-state particle mapping
• recovery testing
• airflow visualization
• worst-case stability checks
Once documented in CCS, dynamic operation within this envelope is GMP-compliant and energy-efficient.
D. Protect the Risk Point, Not the Entire Room
Localized protection often improves sterility while reducing load:
• UDAF at exposure points
• RABS or isolators
• point-of-use HEPAs
• barrier-based layouts
Strategy 2: HVAC Engineered for Minimum Safe Energy
A. Reduce Fan Power First
Fan energy never sleeps. Key levers include:
• high-efficiency fans and EC motors
• low-pressure duct routing
• correct duct velocities
• low initial ΔP filters
• elimination of unnecessary restrictions
Small resistance reductions deliver massive lifecycle savings.
B. Heat Recovery for High Fresh-Air Systems
Sterile and containment blocks with high OA benefit most from:
• segregated plate exchangers
• run-around coils
• chilled/hot water reset strategies
These reduce energy while improving temperature stability.
C. Humidity Control Without Waste
Avoid over-dehumidification followed by reheating by:
• seasonal dewpoint reset
• avoiding unnecessarily tight RH bands
• desiccant or hybrid systems where climate demands
• preventing simultaneous heat–cool loops
D. Validated VFD Dynamic Control
When qualified, dynamic control enables:
• low-occupancy modes
• night setbacks
• process-dependent ACPH reduction
• adaptive pressure balancing
Regulators object to unvalidated change—not energy saving.
Strategy 3: Smart Cleanrooms & Continuous Verification
Traditional cleanrooms are qualified periodically. Smart cleanrooms are verified continuously.
A. Cleanroom Health Index
Combine key indicators into a unified view:
• particle trends
• pressure stability
• airflow balance
• temperature/RH drift
• door-event behavior
• filter ΔP trends
• recurring micro-alarms
This gives QA and Operations a shared control language.
B. Predictive, Condition-Based Maintenance
Data-driven maintenance:
• safely extends filter life
• reduces unplanned shutdowns
• lowers waste
• prevents EMS excursions
C. Digital Evidence for Qualification
Secure trending allows:
• EMS/BMS logs as PQ evidence
• faster RCA
• audit-ready data
• performance-based requalification
Strategy 4: Water, Cleaning & Waste Optimization
A. Validated Low-Water Cleaning
Risk-based foam/spray methods reduce water and chemical load, especially in support zones.
B. CIP/SIP Cycle Tuning
Optimize based on endpoints, not habit:
• rinse-endpoint monitoring
• removal of over-rinse
• heat recovery from effluent
• validated condensate recovery
C. Gowning by Lifecycle
Evaluate reusable vs disposable gowns by:
• contamination risk
• wash energy vs disposal load
• grade-based usage
• smart issue/return tracking
Illustrative Pharma Transformations
Case 1 – ACPH Rationalization:
Validated lower ACPH bands in support rooms reduced fan energy, stabilized pressures, and cut EMS alarms.
Lesson: validated envelopes outperform inherited numbers.
Case 2 – Heat Recovery in Sterile Blocks:
Run-around coils reduced cooling load, stabilized temperatures, and paid back quickly.
Lesson: recovery works best where fresh air is highest.
Case 3 – Barrier Technology Adoption:
RABS/isolators enabled background grade reduction with higher sterility assurance.
Lesson: protect exposure, not address.
Maturity Ladder for Facilities
Compliant baseline
Risk-based efficiency
Smart verified operation
Autonomous control
Most Indian sites are between Levels 1 and 2. Moving to Level 3 depends more on validation confidence and culture than on hardware.
Five Rules for Every Project
Risk defines grade—not tradition
Performance is validated; numbers justified
Air is the costliest utility you manufacture
Continuous verification makes sustainability audit-safe
Lifecycle thinking beats CAPEX fear
The cleanroom of the future is not the biggest or the highest grade—it is the one right-sized by risk, engineered for minimum safe resources, and proven in control every day by data.
Special Thanks:

Narender Pal Singh is Vice President and Site Head – Operations & Projects at Panacea
Biotec Ltd., bringing 25+ years across pharmaceutical engineering, utilities, projects,
sterile/biotech facility management, cleanrooms, and EHS. He has led multiple greenfield and
brownfield transformations focused on contamination control strategy (CCS), operational
reliability, and lifecycle GMP compliance. His work integrates risk-based cleanroom design,
energy-efficient HVAC and predictive maintenance. A regular speaker at industry forums and
universities, he recently conducted a “Factory Fit Assessment” training for the DCVMN, with
delegates from across the globe. Training materials are hosted on the TTT5 Post-Training
Resource Hub on the DCVMN Moodle e-Learning Platform.