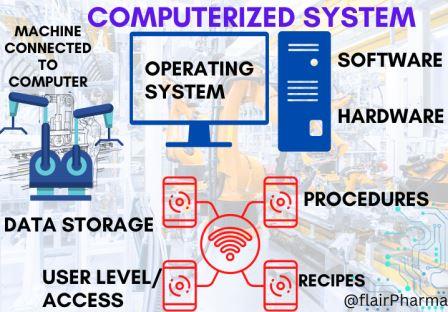
Computer System Validation (CSV): In pharmaceutical industries, every manufacturing plant will run its system, machines, and product in self-driven mode ie. “Automation” and these systems have critical software, computerized controls, and measurements. As a result, regulatory authorities make it mandatory to validate and standardize computer systems in accordance with their standards. So that the worldwide GAMP 5 provides the computer system validation guidelines. 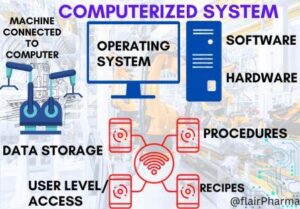
Computer System Validation
Introduction:
Computer System Validation (CSV) is a necessity of every pharmaceutical organization. Nowadays every organization used automation & artificial intelligence in their plant to make their system foolproof and automation needs a computer system for monitoring & controlling the systems. Production equipment as well as utility systems are SCADA-based systems. So nowadays computer system drives the systems with better quality and productivity. The GAMP 5 guidelines give a framework and methodologies to intend and provide an overview of Computer System Validation (CSV) and a road map of the process and steps used in the CSV process.

Key Points of Computer System Validation
Computer System Validation (CSV) is a process used in the pharmaceutical industry to ensure that computer-based systems used for manufacturing, testing, and quality control of pharmaceutical products meet specific requirements. Some key points of computer system validation in pharma are:
- Risk Assessment: The first step in computer system validation is to identify and assess the risks associated with the system. This involves analyzing the system’s criticality, the potential impact on product quality and patient safety, and the likelihood of system failures.
- Validation Plan: A validation plan is developed that outlines the scope of the validation, the approach to be taken, and the responsibilities of each stakeholder involved in the validation process.
- System Requirements: Detailed system requirements are established, which specify the functional and non-functional requirements of the system. These requirements are used as a basis for testing the system’s performance and ensuring that it meets the user’s needs.
- User Acceptance Testing (UAT): User acceptance testing is conducted to ensure that the system meets the user’s requirements and that it is fit for use.
- Installation Qualification (IQ): IQ involves verifying that the computer system and its components have been installed and configured correctly and in accordance with the manufacturer’s specifications.
- Operational Qualification (OQ): OQ involves testing the system’s functionality to ensure that it performs as intended under operational conditions.
- Performance Qualification (PQ): PQ involves testing the system’s performance to ensure that it meets the predefined acceptance criteria.
- Documentation: All validation activities are documented, including test protocols, test results, and validation reports. The documentation is reviewed and approved by authorized personnel.
- Change Control: Change control procedures are implemented to manage any changes to the system, including changes to hardware, software, or configuration.
- Periodic Review: Regular periodic reviews are conducted to ensure that the system continues to meet the user’s needs and that it remains in a validated state.
In conclusion, computer system validation is a critical process in the pharmaceutical industry to ensure that computer-based systems used for manufacturing, testing, and quality control of pharmaceutical products are reliable, robust, and compliant with regulatory requirements.
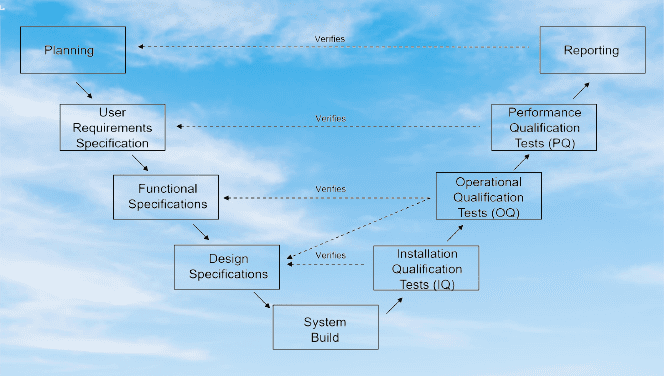
The process to validate the System:
As guided by the GAMP5, computerized systems are validated as per the software and hardware categorization, Also the system complies with USFD 21 CFR part11 Compliances like an electronic record, electronic signature, data audit trails, and data integrity. This guidance forecasts the risk-based approach to validate the system.
As per GAMP5, there are four life cycle phases of the computer system:
- Conceptual details
- Project summary
- Operation and Process
- Retirement or retrievals.
Computerized System Validation» –
Run a CSV based on risk. CSV requires a significant amount of time and IT resources, so it is wise to use a flexible GAMP 5 methodology that makes use of a risk-based evaluation of the system to identify necessary test cases and the best level of testing for each. The important system components that have an impact on quality assurance and regulatory compliance should be the focus of CSV efforts. The advantages of this risk-based approach to CSV include decreased cost, business risk, and effort duration.
Computerized Systems Validation is the confirmation by examination and provision
- Data – All Data shall meet the ALCOA+ requirements:
A -(means) Attributable to the person generating the data.
L – (means) Legible and permanent.
C – (means) Contemporaneous.
O – (means) Original record (or ‘true copy’).
A – (means) Accurate.
‘+’ is referring to the additional measures ensuring that data are Complete, Consistent, Enduring, and Available.
- Data Lifecycle – Life of the data Like Storage, use, data retention, archive/retrieval, and destruction.
- Data Processing – Define the sequence of operations performed and processing parameters.
- Data Review – All data shall process for data review and approval (i.e. audit trails).
- Dynamic Record – All records, such as electronic records, are in a dynamic format.
The list of all the parameters to be taken care of during the computer system validation:
- The Concept Phase: The concept phase is CSV’s initial process, which includes the following steps.
- The Systems Hardware and its Software Categorization.
- GxP Impact Assessment and Risk assessment
- Electronic Records and Electronic Signatures Assessment.
- Project Phase :
- Supplier/vendor assessment at the very start phase with at least three suppliers/vendors are to be asses.
- Risk Management preparation.
- Initial Risk Assessment.
- Functional Risk Assessment
- Validation Plan and its road map
- System Overview with complete details
- User Requirements Specification (URS) preparation & approvals.
- Define Functional Specification
- Define Configuration Specification
- Design Qualification documentation
- Define Functional Design Specification
- Define Hardware Design Specification
- Define Software Design Specification
- Design Review and approval by all stakeholders
- Software Development Program
- Data Migration and Test Plan
- Factory Acceptance Testing Protocol / Report
- Installation Qualification Protocol / Report
- Unit and Integration Testing Protocol / Report
- System Test Protocol / Report
- Perform the Operational Qualification Protocol / Report
- Perform and Acceptance Test Protocol / Report
- Performance Qualification Protocol / Report
- System Operating Procedures / User Manuals
- Training onsite/classroom/on-job
- System Support Plan and define the Service Level Agreement (SLA)
- Handovers and the System Release
- Deployment Planning and Validation Report
- Operation Phase
- Backup and Restore
- Continuity Planning / Testing / Disaster Recovery
- Periodic Review
- Data Archive & Retrieval
- Retirement Phase
- Decommissioning Plan / Report
- Multi-Phase
- Requirements Traceability
- Change Management
- Incident / Deviation Management
- Document Management
- Configuration Management
- Access and Security Management
References:
ISPE GAMP 5, 2008, providing major description on “A Risk-Based Approach to Compliant GxP Computerized Systems”.
Importance of Computer System Validation in pharma
The pharmaceutical industry is highly regulated, and any errors or failures in the manufacturing, testing, or quality control of pharmaceutical products can have serious consequences, including harm to patients, regulatory non-compliance, and financial losses. Computer System Validation (CSV) is important in the pharmaceutical industry for several reasons:
- Ensures Compliance: CSV ensures that computer-based systems used for manufacturing, testing, and quality control of pharmaceutical products comply with regulatory requirements, including FDA, cGMP, and 21 CFR Part 11.
- Minimizes Risks: CSV minimizes the risks associated with computer-based systems by identifying and assessing the potential risks and implementing appropriate controls to manage those risks.
- Ensures Data Integrity: CSV ensures that the data generated by computer-based systems used in the pharmaceutical industry are accurate, complete, and reliable, which is critical to ensuring the safety and efficacy of pharmaceutical products.
- Improves Efficiency: CSV improves the efficiency of the manufacturing, testing, and quality control processes by ensuring that the computer-based systems used in these processes are reliable and operate as intended.
- Improves Product Quality: CSV improves the quality of pharmaceutical products by ensuring that the computer-based systems used for manufacturing, testing, and quality control of pharmaceutical products are accurate and reliable.
- Enhances Reputation: CSV enhances the reputation of pharmaceutical companies by demonstrating their commitment to quality and compliance with regulatory requirements.
In conclusion, Computer System Validation (CSV) is crucial in the pharmaceutical industry as it ensures compliance, minimizes risks, ensures data integrity, improves efficiency, improves product quality, and enhances the reputation of pharmaceutical companies. Failure to validate computer-based systems in the pharmaceutical industry can result in severe consequences, including harm to patients, regulatory non-compliance, and financial losses.
Frequently Asked Questions Regarding CSV:
In India many vendors have the setup for computer system validation if you want to know more about the CSV or related activities please feel free to contact: admin@flairpharma.com