The Annual Product Quality Review (APQR) stands as a cornerstone in the realm of pharmaceuticals, ensuring the maintenance of high-quality standards. APQR is a comprehensive evaluation conducted annually to assess the quality of pharmaceutical products. It serves as a regulatory requirement in many countries and plays a vital role in maintaining product integrity, patient safety, and regulatory compliance.
The primary objective of APQR is to analyze and evaluate various aspects of the pharmaceutical product’s quality throughout its lifecycle. This includes an assessment of manufacturing processes, analytical data, stability studies, and any changes made during the year. By conducting a thorough review, pharmaceutical companies can identify potential risks, deviations, and areas for improvement.
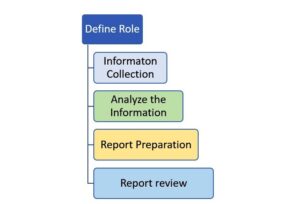
APQR involves a systematic and holistic approach. It begins with the collection of relevant data, such as manufacturing records, analytical results, and customer complaints. The data is then analyzed to identify trends, patterns, and potential quality issues. The review process also includes an assessment of any changes made to the manufacturing process or formulation, as well as an evaluation of product stability and shelf-life data.
One of the key benefits of APQR is its ability to provide insights into the overall performance and quality of pharmaceutical products. By analyzing the collected data, companies can identify areas of strength and weakness, enabling them to take proactive measures for continuous improvement. APQR also helps in identifying potential risks and implementing appropriate corrective and preventive actions to mitigate them.
Furthermore, APQR plays a crucial role in regulatory compliance. Regulatory authorities require pharmaceutical companies to demonstrate a robust quality management system, and APQR serves as evidence of the company’s commitment to maintaining product quality and patient safety. It supports regulatory filings, inspections, and audits by providing comprehensive documentation of the product’s quality profile.
Elements of the Product Quality Review in Pharmaceuticals
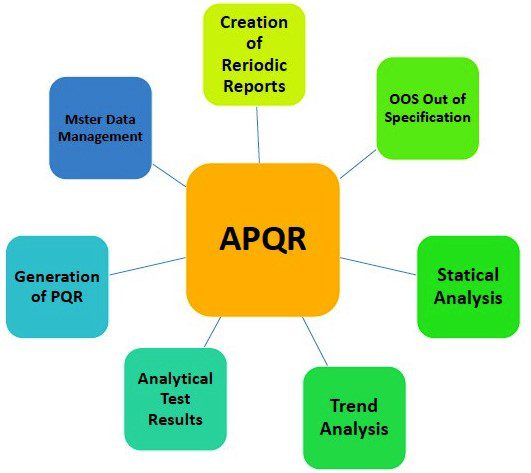
- Manufacturing Processes: Evaluate the adequacy and effectiveness of manufacturing processes, including equipment calibration, maintenance, and cleaning procedures.
- Batch Analysis: Assess the results of batch analysis, including raw materials testing, in-process testing, and finished product testing to ensure compliance with specifications and standards.
- Stability Studies: Review stability data to assess the product’s shelf life, storage conditions, and potential degradation over time.
- Analytical Method Validation: Verify the validation status of analytical methods used for product testing, ensuring they are accurate, reliable, and suitable for their intended purpose.
- Complaints and Adverse Events: Analyze customer complaints and reported adverse events to identify any recurring issues or potential risks to patient safety.
- Deviations and Corrective Actions: Evaluate the documentation of deviations encountered during manufacturing and the effectiveness of implemented corrective and preventive actions.
- Change Control: Assess the management of changes to processes, equipment, formulations, or suppliers to ensure proper evaluation, approval, and implementation, as well as their impact on product quality.
- Regulatory Compliance: Ensure compliance with applicable regulatory requirements, including Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and relevant guidelines from regulatory authorities.
- Documentation and Record Keeping: Review the accuracy, completeness, and accessibility of documentation and records related to the product’s manufacturing, testing, and quality assurance.
- Trend Analysis: Conduct trend analysis of quality data over time to identify patterns, variations, and potential areas for improvement.
- Risk Assessment: Perform a risk assessment to identify and evaluate potential risks associated with the product, manufacturing processes, and supply chain, and implement appropriate risk mitigation strategies.
- Supplier Performance: Evaluate the performance of key suppliers, including raw material suppliers, contract manufacturers, and testing laboratories, to ensure their compliance with quality standards.
- Regulatory Filings: Review the accuracy and completeness of regulatory filings, such as Drug Master Files (DMFs) or New Drug Applications (NDAs), to ensure alignment with the product’s quality data.
- Continuous Improvement: Identify opportunities for continuous improvement in product quality, processes, and systems to enhance overall efficiency and effectiveness.
It’s important to note that this list may vary depending on specific regulatory requirements, company policies, and the nature of the pharmaceutical product being reviewed.
Benefits of Product Quality Review (PQR)
| Benefits of PQR |
|---|
| Ensures compliance with regulatory standards and guidelines |
| Identifies and mitigates risks to patient safety and product quality |
| Enhances product performance and reliability |
| Facilitates continuous improvement in manufacturing processes |
| Detects and resolves deviations and non-conformities |
| Improves customer satisfaction and trust in the product |
| Supports data-driven decision making for quality enhancements |
| Provides insights for optimizing manufacturing efficiency |
| Identifies trends and patterns in quality data |
| Helps in maintaining consistent product quality throughout the lifecycle |
| Supports regulatory filings and inspections |

