Aseptic areas are specialized zones within healthcare facilities or pharmaceutical manufacturing sites that are designed and maintained to prevent the introduction and spread of microorganisms. These areas play a critical role in maintaining sterility and reducing the risk of infections during medical procedures or the production of sterile products.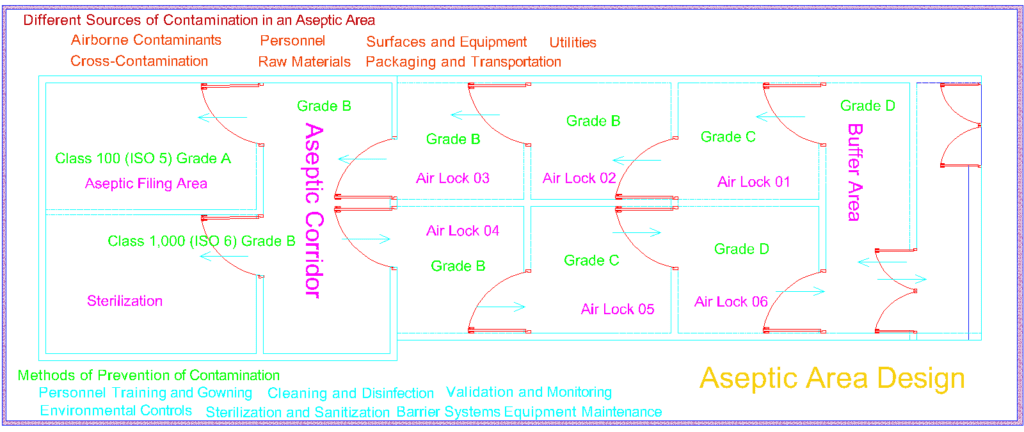
Aseptic areas are characterized by strict protocols, procedures, and controls to ensure a microbiologically clean environment. They are equipped with features such as laminar airflow systems, high-efficiency particulate air (HEPA) filters, and controlled air pressure differentials to minimize the presence of airborne contaminants.
In healthcare settings, aseptic areas are commonly found in operating rooms, surgical suites, and specialized units like intensive care units (ICUs). These areas are designed to create a sterile environment for surgical procedures, invasive interventions, and patient care activities. Healthcare professionals in aseptic areas follow rigorous hand hygiene practices, wear sterile gowns and gloves, and adhere to aseptic techniques to prevent the transmission of pathogens.
In pharmaceutical manufacturing, aseptic areas are crucial for the production of sterile drugs, parenteral medications, and medical devices. These areas are designed to prevent microbial contamination during the manufacturing, filling, and packaging processes. Stringent controls are implemented to ensure the sterility of materials, equipment, and personnel involved in production.
The design and operation of aseptic areas are guided by regulatory standards and industry best practices. Regular monitoring, environmental sampling, and validation activities are conducted to verify the effectiveness of the aseptic processes and maintain compliance with regulatory requirements.
The primary goal of aseptic areas is to provide a controlled and sterile environment, ensuring the safety and well-being of patients and the quality of manufactured products. By implementing aseptic practices and maintaining high levels of cleanliness, aseptic areas contribute to reducing the risk of infections, improving patient outcomes, and ensuring the integrity of sterile products in the healthcare and pharmaceutical industries.
Aseptic Area Design
Designing an aseptic area entails a meticulous examination of multiple dimensions. Architectural elements, such as room layout, air pressure differentials, and material compatibility, intertwine to create a harmonious ecosystem that prevents the intrusion of contaminants. The complexity lies in achieving a seamless integration of physical barriers, personnel flows, and equipment placement, where every element plays a crucial role in maintaining the sanctity of the sterile environment
Aseptic definition
Aseptic, in the context of pharmaceutical manufacturing and healthcare settings, refers to the state of being free from microorganisms, particularly pathogenic ones. It pertains to the measures and practices employed to prevent contamination and maintain sterility in various processes, environments, and procedures. Aseptic techniques and protocols involve meticulous disinfection, sterilization, and the use of sterile equipment and materials to ensure the safety and integrity of pharmaceutical products, medical devices, and invasive procedures. The aseptic approach aims to minimize the risk of infections, promote patient safety, and uphold the highest standards of quality in the healthcare and pharmaceutical industries.
Aseptic cleanroom
An aseptic cleanroom refers to a controlled environment designed to maintain high levels of cleanliness and sterility in order to prevent the introduction or proliferation of microorganisms. It is a specialized area within industries such as pharmaceuticals, biotechnology, medical devices, and healthcare, where the production, manufacturing, or handling of sterile products takes place.
Aseptic cleanrooms are constructed with meticulous attention to detail, incorporating features such as air filtration systems, controlled airflows, and strict adherence to cleanliness protocols. These cleanrooms are typically equipped with high-efficiency particulate air (HEPA) filters that remove airborne particles, including bacteria and viruses, to maintain a highly clean and sterile environment.
Within the aseptic cleanroom, personnel undergo rigorous gowning procedures and follow specific aseptic techniques to minimize the risk of contamination. This may include wearing sterile garments, gloves, and masks, and taking appropriate measures to prevent the shedding of particles or microorganisms.
The purpose of an aseptic cleanroom is to create a controlled environment that meets specified cleanliness standards, such as ISO Class 5 or higher, as defined by international standards organizations. This ensures that products manufactured or handled in the cleanroom are free from harmful microorganisms, thus reducing the risk of contamination and maintaining product integrity.
Aseptic techniques
Aseptic cleanrooms play a vital role in industries where sterility is paramount, as they provide a controlled environment that supports the production of safe and sterile products. By implementing stringent cleanliness and aseptic protocols, aseptic cleanrooms contribute to the overall quality, safety, and efficacy of products in the healthcare and pharmaceutical industries.
| Aseptic Area Type | Grade |
|---|---|
| Class 100 (ISO 5) | A |
| Class 1,000 (ISO 6) | B |
| Class 10,000 (ISO 7) | C |
| Class 100,000 (ISO 8) | D |
These grades provide a standardized system to evaluate the cleanliness and level of control required in aseptic areas. The lower the grade number, the stricter the cleanliness requirements and the higher level of control needed to maintain the sterility of the area.
Different Sources of Contamination in an Aseptic Area
An aseptic area, despite its stringent controls and protocols, can still be susceptible to various sources of contamination. Identifying and addressing these potential sources is crucial to maintaining the sterility and integrity of the environment. Here are some common sources of contamination in an aseptic area:

- Airborne Contaminants: Microorganisms and particles suspended in the air can pose a significant risk to the sterility of the area. These contaminants may include bacteria, fungi, viruses, dust, and other airborne particles. Proper air filtration systems, such as high-efficiency particulate air (HEPA) filters, are essential in removing or reducing airborne contaminants.
- Personnel: Human operators working within the aseptic area can inadvertently introduce contaminants. Skin shedding, respiratory emissions, and improper hand hygiene are potential sources of microbial contamination. Strict adherence to gowning procedures, appropriate use of personal protective equipment (PPE), and rigorous training in aseptic techniques help minimize personnel-related contamination risks.
- Surfaces and Equipment: Surfaces, including countertops, workstations, and equipment, can harbor microorganisms and particles. Insufficient cleaning and disinfection practices, as well as improper handling of materials, can contribute to surface contamination. Regular cleaning and disinfection using appropriate agents and techniques are necessary to maintain a clean and sterile environment.
- Raw Materials: The quality and sterility of raw materials, such as ingredients, components, and packaging materials, can impact the contamination levels within the aseptic area. Contamination can occur during the transportation, storage, or handling of these materials. Rigorous testing, proper storage conditions, and validated sterilization processes are essential to ensure the sterility of raw materials.
- Utilities: Water, compressed air, and other utilities used within the aseptic area can potentially introduce contaminants if they are not appropriately purified or controlled. Implementing robust purification systems and regularly monitoring the quality of utilities are crucial to minimizing the risk of contamination.
- Cross-Contamination: Transferring contaminants between different areas or equipment within the aseptic environment can lead to cross-contamination. This can occur through improper cleaning, inadequate separation of processing areas, or improper handling of materials. Strict segregation of processes, dedicated equipment, and thorough cleaning procedures are essential in preventing cross-contamination.
- Packaging and Transportation: Packaging materials and transportation containers used for finished products can introduce contaminants if they are not properly cleaned, sterilized, or handled. Ensuring proper cleaning, sterilization, and appropriate packaging methods are essential to maintain the sterility of products during storage and transportation.
Regular monitoring, environmental sampling, and robust quality control measures are vital for identifying and mitigating potential sources of contamination in an aseptic area. By addressing these sources and implementing effective preventive measures, the risk of contamination can be minimized, allowing for the production of safe and sterile products within the aseptic environment.
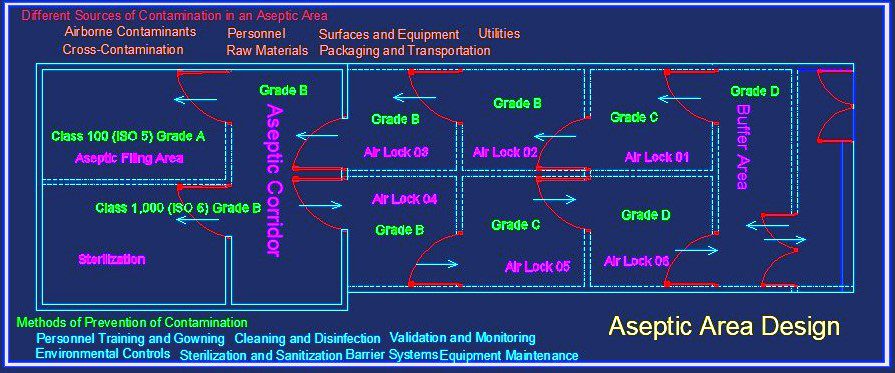
Methods of Prevention of Contamination
Preventing contamination is of paramount importance in maintaining the sterility and integrity of an aseptic environment. Several methods and practices are employed to minimize the risk of contamination. Here are some key methods of contamination prevention:
- Personnel Training and Gowning: Thorough training of personnel on aseptic techniques, proper gowning procedures, and hand hygiene is essential. Education and regular reinforcement of good aseptic practices help reduce the potential for contamination caused by human factors.
- Environmental Controls: Implementing robust environmental controls is crucial. This includes maintaining appropriate air quality through the use of high-efficiency particulate air (HEPA) filters, controlling temperature and humidity, and monitoring air pressure differentials to prevent the entry of contaminants from surrounding areas.
- Cleaning and Disinfection: Regular and effective cleaning and disinfection of surfaces, equipment, and workspaces help eliminate or reduce microbial contaminants. Using appropriate disinfectants and following validated cleaning protocols are essential for ensuring a clean and sterile environment.
- Sterilization and Sanitization: Proper sterilization and sanitization of equipment, instruments, and materials play a critical role in contamination prevention. Employing validated sterilization methods, such as steam sterilization or sterilizing filtration, helps eliminate microorganisms from critical components.
- Barrier Systems: Implementing physical barriers, such as laminar flow hoods, isolators, or cleanrooms, can provide an additional layer of protection against contamination. These barriers create controlled environments that minimize the entry of contaminants and help maintain sterility during critical operations.
- Material Control: Implementing stringent material control measures, including thorough inspection and testing of incoming materials, helps ensure the quality and sterility of raw materials, components, and packaging. Proper storage and handling practices should also be followed to prevent contamination during material transportation and usage.
- Equipment Maintenance: Regular maintenance and calibration of equipment, such as cleanrooms, air handling units, and filtration systems, are crucial for optimal performance and contamination prevention. Monitoring equipment performance and promptly addressing any issues or malfunctions are essential to maintain the integrity of the aseptic environment.
- Validation and Monitoring: Regular validation and monitoring activities, including environmental monitoring, personnel monitoring, and process validation, provide ongoing assurance of the effectiveness of contamination prevention measures. These activities help identify potential sources of contamination and allow for timely corrective actions to be taken.
Clean Area Classification
| Clean Area Classification | Maximum Particles per Cubic Meter (≥0.5 μm) | Maximum Particles per Cubic Meter (≥5 μm) |
|---|---|---|
| ISO 1 | 10 | 2 |
| ISO 2 | 100 | 20 |
| ISO 3 | 1,000 | 400 |
| ISO 4 | 10,000 | 2,000 |
| ISO 5 | 100,000 | 20,000 |
| ISO 6 | Not specified | 200,000 |
| ISO 7 | Not specified | 352,000 |
| ISO 8 | Not specified | 3,520,000 |
These classifications provide a standardized system to evaluate and maintain the cleanliness of controlled environments. The lower the ISO classification number, the stricter the cleanliness requirements, with ISO 1 being the cleanest and ISO 8 being the least stringent.
It’s important to note that specific industries or applications may have additional requirements or guidelines beyond the ISO classifications
Limits for Microbial Contamination in the Operation State (Average Values)
| Grade | Air Sample, cfu/m3 | Settle Plates (Diameter 90mm), cfu/4 hours (a) | Contact Plates (Diameter 55mm), cfu/plate | Glove Print, 5 Fingers cfu/glove |
| A | 1 | < 1 | < 1 | < 1 |
| B | 10 | 5 | 5 | 5 |
| C | 100 | 50 | 25 | – |
| D | 200 | 100 | 50 | – |
Aseptic Techniques and Aseptic manufacturing process
| Aseptic Manufacturing Grade | Process Steps |
|---|---|
| Grade A | – Sterilization of critical equipment and materials. |
| – Aseptic gowning and hand hygiene. | |
| – Preparation of sterile products. | |
| – Filling and sealing of containers under aseptic conditions. | |
| – Sterilization of final product containers. | |
| – Transfer of sterile products to Grade B area. | |
| Grade B | – Material transfer from Grade A area. |
| – Aseptic processing of materials. | |
| – Aseptic filling and packaging of products. | |
| – Environmental monitoring and control. | |
| Grade C | – Material transfer from Grade B area. |
| – Controlled processing of materials. | |
| – Packaging and labeling operations. | |
| – Environmental monitoring and control. | |
| Grade D | – Material transfer from Grade C area. |
| – Non-sterile material handling and preparation. | |
| – Environmental monitoring and control. |
Frequently Asked Questions
What is aseptic area and sterile area?
| Aspect | Aseptic Area | Sterile Area |
|---|---|---|
| Definition | An area designed to maintain a controlled environment to minimize the risk of microbial contamination | An area that has been completely free from viable microorganisms, ensuring a state of sterility |
| Purpose | To provide a controlled environment for the production of sterile products | To ensure the sterility of products, materials, or processes |
| Microbial Control | Focuses on minimizing the introduction, proliferation, and dissemination of microorganisms | Focuses on eliminating all forms of viable microorganisms |
| Classification | Can have different classification levels (e.g., ISO 5, ISO 6, ISO 7) based on particle count limits | Not classified based on particle count limits |
| Operations | Typically used for aseptic processing, filling, and packaging of sterile products | Used for operations that require an absolute sterile environment, such as aseptic filling or surgeries |
| Control Measures | Requires strict adherence to aseptic techniques, gowning, and environmental monitoring | Requires rigorous sterilization procedures, such as steam sterilization or sterilizing filtration |
| Contamination Risk | A controlled environment that minimizes the risk of contamination but does not guarantee sterility | An environment that has achieved a state of sterility, ensuring no viable microorganisms are present |
What is clean room and aseptic area?
Answer: Cleanroom: A cleanroom is a controlled environment that is designed to minimize the presence of airborne particles, contaminants, and other factors that could adversely affect the quality of products or processes being carried out within it. Cleanrooms are typically used in industries such as pharmaceuticals, biotechnology, electronics, and healthcare, where the control of particulate and microbial contamination is critical. They are constructed and maintained to meet specified cleanliness levels by controlling factors such as air filtration, airflow patterns, temperature, humidity, and pressure differentials.
Aseptic Area: An aseptic area refers to a designated space within a cleanroom or other controlled environment that is specifically designed and maintained to provide a sterile environment for the handling, processing, and packaging of sterile products. Aseptic areas are utilized in industries such as pharmaceutical manufacturing, biotechnology, and healthcare, where it is essential to maintain sterility and prevent microbial contamination. Aseptic areas employ stringent protocols, specialized equipment, and procedures to minimize the introduction, proliferation, and dissemination of microorganisms. This includes measures such as proper gowning, sterile processing techniques, environmental monitoring, and validation of processes to ensure the integrity and sterility of the products being produced.
In summary, a cleanroom is a controlled environment that aims to minimize airborne particles and contaminants, while an aseptic area is a specific part of a cleanroom or controlled environment that focuses on maintaining sterility for the handling and processing of sterile products.
How do you classify an aseptic area?
Answer: An aseptic area is classified based on the level of cleanliness and control it maintains in terms of airborne particles. The classification of an aseptic area is typically determined using international standards such as ISO 14644-1:2015, which provides guidelines for cleanrooms and controlled environments. The classification is primarily based on the maximum allowable concentration of particles of specific sizes within the area.
The classification is denoted by an ISO class number, where a lower number indicates a higher level of cleanliness. The most commonly used particle sizes for classification are ≥0.5 μm and ≥5 μm. The particle count limits for each size determine the ISO class of the aseptic area.
The classification process involves measuring the concentration of particles within the specified size range using particle counters. The sampling is done at various locations and times to ensure representative results. The collected data is then compared to the defined ISO classification limits to determine the appropriate class for the aseptic area.
For example, a typical classification system may include ISO classes such as ISO 5, ISO 6, ISO 7, and ISO 8. ISO 5 represents the cleanest and most stringent classification, indicating a very low concentration of particles, while ISO 8 represents a less stringent classification with a higher allowable particle count.
It’s important to note that the specific classification requirements may vary depending on industry regulations, standards, and specific applications. Compliance with the defined classification standards is essential to ensure the appropriate level of cleanliness and control in an aseptic area.
What is the purpose of an aseptic area in pharmaceutical manufacturing?
Answer: The purpose of an aseptic area is to maintain a sterile environment for the production of sterile pharmaceutical products, minimizing the risk of microbial contamination.
What are the key components of a typical aseptic area?
Answer: A typical aseptic area includes laminar airflow systems, high-efficiency particulate air (HEPA) filters, controlled air pressure differentials, sterile gowning areas, and dedicated equipment for aseptic processing.
What is the importance of environmental monitoring in an aseptic area?
Answer: Environmental monitoring helps assess the level of cleanliness and control within the aseptic area by monitoring airborne particles, microbial contamination, and other potential sources of contamination.
What are the critical factors in maintaining sterility within an aseptic area?
Answer: Critical factors include proper personnel training in aseptic techniques, rigorous gowning and hand hygiene practices, effective cleaning and disinfection protocols, thorough material control, and regular maintenance and validation of equipment.
How is the cleanliness of an aseptic area measured and classified?
Answer: The cleanliness of an aseptic area is measured through particle counts at different sizes using particle counters. It is then classified based on the ISO classification system, which specifies maximum allowable particle limits for different particle size ranges.
What are the challenges in maintaining an aseptic environment?
Answer: Challenges include controlling airborne contaminants, minimizing personnel-related contamination, addressing surface and equipment contamination, ensuring the quality and sterility of raw materials, and maintaining proper validation and monitoring procedures.
What are the commonly used disinfectants in an aseptic area?
Answer: Commonly used disinfectants include alcohol-based solutions, hydrogen peroxide, quaternary ammonium compounds, and sporicidal agents. The selection depends on the type of surface, efficacy against target microorganisms, and compatibility with the aseptic environment.
How are aseptic techniques applied during filling and packaging operations?
Answer: Aseptic techniques during filling and packaging operations involve using sterile equipment, maintaining a sterile field, monitoring air quality, employing sterile gowning and gloving procedures, and ensuring proper aseptic connections and closures.
What are the risks associated with improper aseptic practices in an aseptic area?
Answer: Improper aseptic practices can lead to microbial contamination of products, compromised sterility, increased risk of infections in healthcare settings, decreased shelf life of pharmaceutical products, and non-compliance with regulatory requirements.
How is the qualification of personnel in an aseptic area ensured?
Answer: Personnel in the aseptic area undergo comprehensive training on aseptic techniques, gowning procedures, hand hygiene, cleanroom behavior, and contamination control. Qualification may involve written assessments, practical evaluations, and ongoing training to ensure adherence to aseptic practices.


3 thoughts on “Aseptic Area 2025”