There are Different sources of Contamination in Aseptic Area. An aseptic area is a premise in a clean area, designed, constructed, serviced, and used with the intention of preventing microbial contamination of the product. It is essential to identify the sources from which the contaminants can enter the aseptic area in order to avoid the contamination of products under operation. These sources of Contamination in Aseptic Area cover with a CCS Contamination control strategy and the Data integrity in CCS. 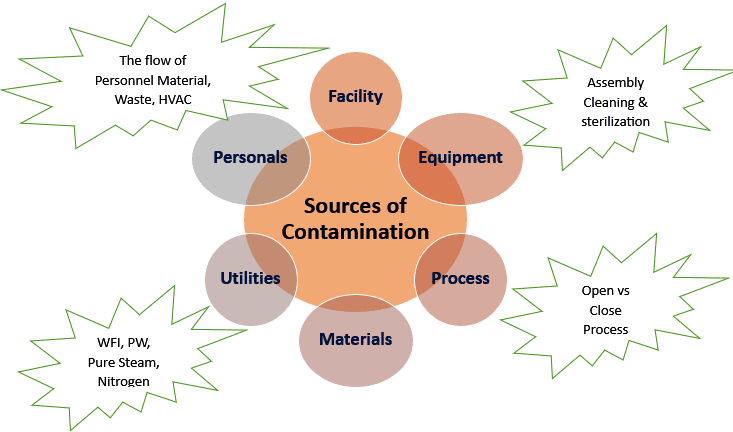
The Major Sources of Contaminations in Sterile Area
The essential Different sources of Contamination in Aseptic Area are as follows.
➢ Personnel.
➢ Buildings and Facilities.
➢ Equipment and Utensils.
➢ Raw Materials.
➢ Manufacturing Process.
➢ Heating, Ventilation, and Air Conditioning (HVAC) System.
➢ Utilities.
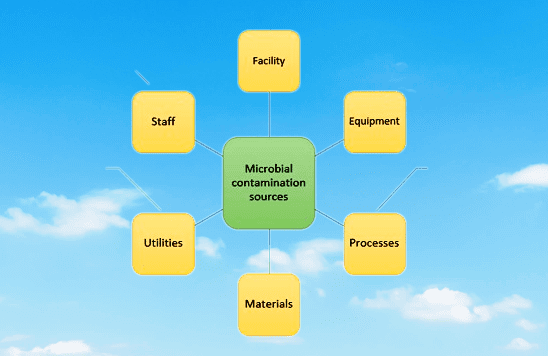
1) Staff or Personnel:•
The personnel working in an aseptic area can be a potential source of microbial contamination, due to the following reasons, these are the sources of Contamination In Aseptic Area :
- Inadequate training,
- Direct contact between the hands and materials.
- Improper hygiene,
- Unauthorized personnel entering the facility, (This is why educational visits are denied in sterile areas).
- Without wearing enough safety gear, Eating, drinking, or smoking inside the processing and storage facilities.
These are the main sources of Contamination in Aseptic.
2) Construction and Infrastructure:•
These can also play a significant role in microbial contamination because of the following factors, this is one of the major sources of Contamination In Aseptic Area:
- Inadequate size and organization of the space can result in selection errors (such as mix-ups or cross-contamination between consumables, raw materials, in-process materials, and finished products).
- Poor filth and pest controls.
- Rough floors, walls, and ceilings.
- Absence of air filtration systems.
- Inadequate lighting.
These are the main sources of Contamination in Aseptic.
3) Equipment and Utensils:•
They are familiar sources of microbial contamination due to the following reasons, this is also the main sources of Contamination In Aseptic Area:
- Unsuitable design, size, corrosion-causing materials, and/or adulteration with lubricants, coolants, dirt, and sanitizing agents.
- Insufficient sanitization and cleaning.
- Due to the way they are designed, cleaning and maintenance are ineffective.
- Inadequate calibration, and inconsistent servicing.
- Making use of broken machinery.
4) Raw Materials (RM):•
They are employed in the manufacturing process and are regarded as possible Sources Of Contamination In Aseptic Area for the following reasons:
- Poor handling and storage cause confusion or selection problems.
- Contamination is caused by microbes or chemicals.
- Degradation is brought on by harsh environmental circumstances (like heat, cold, sunlight, moisture, etc.).
- Inaccurate labeling.
- incorrect testing and sampling,
- Utilizing resources that don’t adhere to the standards of acceptance.
5) Production Method:•
The following factors can all contribute to microbial contamination of raw materials, intermediates, or packaging materials during the manufacturing process these are sources of Contamination In Aseptic Area:
- Lack of facilities necessary for producing a single product.
- Inadequate cleaning between batches to reduce the number of product changes.
- Using an open production system to expose the product to the room’s environment.
- Inadequate zoning.
- Not having an area line clearing between each batch and after each cleaning operation (as per the approved procedures).
- No labels indicating the cleaning status of any tools or supplies utilized in the manufacturing plant.
6) Heating, Ventilation, and Air Conditioning (HVAC) System:•
A poor HVAC system can spread toxins throughout the production facility and be a source of microbial development and the main sources of contamination In Aseptic Area. The following causes for this are:
- Organic compounds collect at or in the vicinity of HVAC air intakes,
- insufficient air filtering system,
- Insufficient pressure differentials, which result in flow reversal,
- Improper fresh-to-recirculated air ratio,
- Access to ventilation dampers and filtration from outside the manufacturing areas is not possible.
- Non-directional airflow in the main packing or production regions.
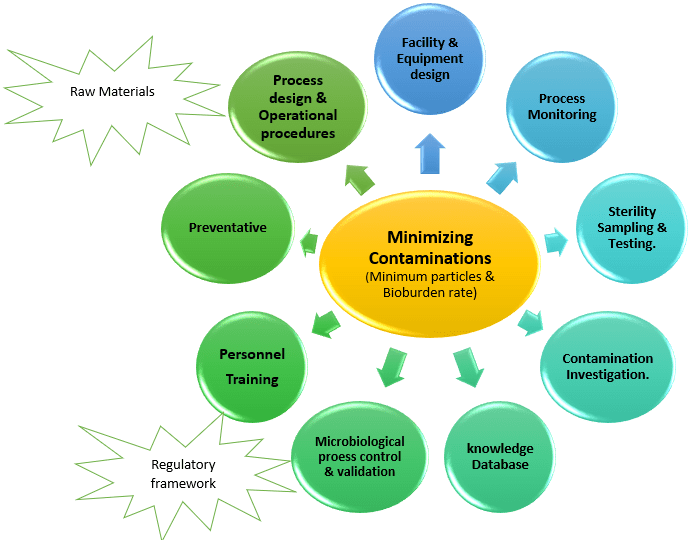
Techniques for Preventing Contamination:
Sources Of Contamination In Aseptic Area Vs its Prevtion as per following aspects:
1) Personnel:
- Unauthorized personnel should not be permitted access to production areas, and only trained employees should be permitted entry.
- Maintaining adequate staff hygiene is important.
- To prevent their actions from impairing the quality of the product, the staff should receive appropriate and regular training in cleanliness.
- Workers should wear protective equipment before entering the manufacturing area (over-garments, hair cover, beard or mustache cover, and overshoes).
- Employees should refrain from contacting any exposed products or equipment that has come into contact with the product with their bare hands.
2) Manufacturing Buildings and Facilities:
- Air filtration and air change rates should be regulated to achieve the designated cleanroom class.
- Differential air pressures in aseptic rooms should be higher than those in the nearby controlled areas.
- Over crucial areas, a unidirectional (laminar flow) airflow should be maintained at a sufficient velocity to sweep debris out of the filling/closing area.
- The humidity and ambient temperature shouldn’t be excessive.
- It is recommended to employ RABS (Restricted Access Barrier System), ventilated cabinets, isolator systems, etc.
- Door barriers and UV airlocks are used to divide the risky locations.
- The isolator/RABS should be used for any open-container processing.
- When compared to the manufacturing area corridor, the air pressure in the changing room should be negative, but when compared to the external surrounding areas, it should be positive.
- For ease of maintenance, the ventilation dampers, specifically made filters, and other services should be placed in service voids or service corridors that are accessible from outside the manufacturing areas.
- In the right places, an impermeable barrier should be employed to avoid cross-contamination between the two zones.
- To stop contaminants produced inside the room from spreading, HVAC air distribution components need to be used.
- This facility must be maintained, checked for viable and non-viable particles on all surfaces, and recertified every six months.
3) Access to Locations
- Unauthorized personnel should not have access to the production, packaging, and quality control facilities.
- Employees should only enter these locations through changing rooms.
- Certain routes should be used to access the materials (generally airlocks).
4) Construction Requirements:
- Ceilings, walls, and flooring should all be smooth, uncracked, and easy to clean.
- To prevent the buildup of dust and microbiological pollutants, windows or viewing panels should be sealed, closed, and secured with wall panels.
5) Cleaning and Disinfection:
- The areas should periodically be cleaned and disinfected.
- To reduce health concerns, cleaning chemicals of the correct grade should be used.
- While cleaning, factors including contact time, application, temperature, mechanical action, and the chemistry of cleaning agents should be considered.
- Cleaning solutions shouldn’t be used directly on the product.
- Cleaning procedures should be validated in order to demonstrate that microbiological contamination is adequately controlled by the procedure.
- Removing the bioburden by dry heat sterilization.
6) Utility Services:
- When making products, pharmaceutical-grade water that is microbiologically inspected and controlled should be utilized.
- To clean and sanitize production tools and equipment, as well as to supply autoclaves and humidification, pure, additive-free steam should be utilized.
Frequently Asked Questions Sources Of Contamination In Aseptic Area
Which of the following is the main source of contamination in aseptic area?
Answer: The introduction of germs into pharmaceutical finished goods is known as pharmaceutical contamination. The following factors might contribute to microbial contamination in pharmaceutical products: raw materials used during production, environmental sources, cleaning tools, packaging, frequently reused containers, repackaging of goods, processing, storage, and transportation.
Pharmaceutical items are susceptible to microbial deterioration due to a variety of factors, such as the quantity and kind of contaminant inoculums, pH, moisture content, storage temperature, packaging design, etc. The proper use and storage of preservatives can successfully avoid pharmaceutical contamination. Preservatives that are frequently utilized include citric acid, benzoic acid, salicylic acid, benzalkonium chloride, phenolic compounds, etc.
You may also read books on the sources of Contamination in Aseptic Area: Integrated Pharmaceutical micro contamination control and Cleanrooms.
if you have any dought on any concept regarding sources of contamination in aseptic area then please mail us at: admin@flairpharma.com

2 thoughts on “Different Sources of Contamination in Aseptic Area 2023”