Hold Time Study refers to a series of controlled experiments conducted within the pharmaceutical sector to assess the stability and viability of a product over a defined period of time. These studies involve subjecting pharmaceutical formulations, whether active ingredients or final products, to predetermined storage conditions to ascertain how they fare in terms of quality, efficacy, and safety as time progresses.
Hold Time Studies provide empirical data that regulatory authorities require for product approval, ensuring that the products reaching patients are safe and effective. In essence, Hold Time Studies provide a window into the future, enabling pharmaceutical organizations to predict the stability and quality of their products over extended periods. By simulating real-world conditions, these studies contribute to optimal manufacturing processes, regulatory compliance, and, most importantly, the well-being of patients relying on these pharmaceutical interventions.
Importance of Hold Time Study in Pharmaceutical Organizations
Hold time studies play a pivotal role within the pharmaceutical sector, offering insights and safeguards that contribute to the industry’s integrity and efficiency. Here are the key reasons why hold time studies hold such significance:
- Ensuring Product Integrity: Hold time studies verify the stability of pharmaceutical products over extended periods. This ensures that products remain effective, safe, and suitable for patient use, safeguarding both consumer health and the pharmaceutical company’s reputation.
- Regulatory Compliance: Regulatory agencies require thorough documentation of a product’s stability and shelf life. Hold time studies provide empirical data that demonstrate compliance with these regulations, preventing legal and financial repercussions.
- Optimizing Production Processes: By determining the optimal hold time for each product, organizations can streamline their manufacturing processes. This reduces waste, enhances resource allocation, and contributes to cost-effectiveness.
- Risk Mitigation: Hold time studies identify potential risks associated with product degradation or instability. This proactive approach enables companies to take corrective measures before issues arise, mitigating the likelihood of recalls or market withdrawals.
- Quality Control: Monitoring a product’s stability under various conditions allows companies to establish quality control standards. This ensures that products consistently meet predefined criteria, maintaining the organization’s commitment to quality.
- Informed Decision-Making: Hold time study data aids in making informed decisions about packaging, labeling, and storage requirements. This knowledge prevents issues arising from inappropriate storage conditions or inadequate packaging materials.
- Research and Development: Data from hold time studies provides valuable insights during the research and development phase. It guides the formulation of products that exhibit greater stability and longevity, leading to enhanced patient outcomes.
- Supply Chain Management: Hold time studies contribute to effective supply chain management. Manufacturers can accurately project shelf life, reducing the risk of products expiring before reaching end-users.
- Market Competitiveness: Organizations with robust hold time study practices gain a competitive edge. Demonstrating the stability and reliability of products enhances consumer trust and bolsters the organization’s standing in the market.
- Continuous Improvement: Hold time studies promote a culture of continuous improvement. Regularly revisiting and updating these studies enables companies to adapt to evolving regulations, technology, and best practices.
- Global Reach: In the interconnected global market, products often cross borders. Hold time studies facilitate international trade by providing standardized stability data that regulatory bodies across different countries can recognize.
- Confidence in Innovation: When introducing novel formulations or manufacturing processes, hold time studies offer a scientific basis for confidently integrating innovation while maintaining product stability and safety.
In summation, hold time studies serve as a cornerstone of pharmaceutical organizations, intertwining scientific rigor with operational excellence. Their multifaceted benefits span from regulatory compliance to cost efficiency, ultimately contributing to the overarching mission of delivering safe and effective healthcare solutions to patients worldwide.
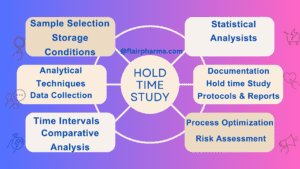
Elements of Hold Time Studies in the Pharmaceutical Industry
Hold Time Study in the pharmaceutical industry encompass a range of meticulously orchestrated components that work in concert to unveil the stability and integrity of pharmaceutical products over time. These elements ensure that products maintain their efficacy, safety, and quality throughout their lifecycle. Here are the key components of Hold Time Study:
- Sample Selection: Hold Time Study begins with the careful selection of representative samples from the pharmaceutical batch. These samples should accurately reflect the entire production lot and its composition.
- Storage Conditions: Hold Time Study involve subjecting samples to specific storage conditions that mimic real-world scenarios. Variables such as temperature, humidity, and light are meticulously controlled to replicate different environmental exposures.
- Time Intervals: Samples are withdrawn from storage at predefined intervals. These intervals are strategically chosen to capture changes in the product’s properties over time, ranging from days to months or even years.
- Analytical Techniques: A suite of analytical methods is employed to evaluate the samples. Techniques such as spectroscopy, chromatography, and molecular assays provide insights into potency, degradation, and other attributes.
- Data Collection: Hold Time Study generate a wealth of data, including quantitative measurements of chemical composition, physical attributes, and performance. This data is collected and recorded for subsequent analysis.
- Comparative Analysis: The collected data is compared against established quality standards and regulatory guidelines. Deviations from expected values may indicate degradation or changes in the product.
- Statistical Analysis: Statistical methods are often employed to discern patterns and trends within the data. This analysis aids in making informed decisions about product stability and shelf life.
- Documentation: A comprehensive record of the entire study is maintained, including details of sample collection, storage conditions, analytical procedures, and results. This documentation is crucial for regulatory compliance and audit purposes.
- Evaluation and Conclusion: At the study’s conclusion, the collected data is meticulously evaluated to determine the product’s stability profile. This assessment informs decisions about the product’s recommended shelf life and storage conditions.
- Regulatory Submission: The insights gleaned from Hold Time Study are pivotal for regulatory submissions. They provide empirical evidence of product stability and serve as a foundation for obtaining approvals from regulatory bodies.
- Process Optimization: Hold Time Study can also guide process optimization efforts. By identifying critical points of degradation, manufacturers can refine production processes to enhance product stability.
- Risk Assessment: Hold Time Study enable organizations to assess potential risks associated with product degradation. This proactive approach aids in risk mitigation strategies.
In the intricate symphony of pharmaceutical development and production, Hold Time Study compose a vital movement. They empower pharmaceutical organizations to ascertain the temporal intricacies that shape product stability and patient safety. By harmonizing these elements, the industry ensures that the medical interventions it delivers stand the test of time, upholding the promise of health and well-being.
Advantages and Disadvantages of Hold Time Study in the Pharmaceutical Industry:
| Advantages of Hold Time Study | Disadvantages of Hold Time Study |
|---|---|
| 1. Product Stability – Ensures products maintain efficacy and quality over extended periods. | 1. Resource Intensive – Hold Time Study requires time, equipment, and personnel resources. |
| 2. Regulatory Compliance – Provides essential data for regulatory submissions and approvals. | 2. Prolonged Timelines – These studies can extend production timelines due to required waiting periods. |
| 3. Quality Assurance – Establishes reliable shelf lives, ensuring consistent product quality. | 3. Cost Implications – The resources required for these studies can add to production costs. |
| 4. Risk Mitigation – Identifies potential degradation risks, enabling proactive corrective actions. | 4. Complex Analysis – Interpreting the data can be intricate, necessitating skilled professionals. |
| 5. Cost Efficiency – Optimizes manufacturing processes, reducing waste and resource usage. | 5. Limited Real-Time Data – Studies provide insights on stability, but real-time data is unavailable. |
| 6. Informed Decisions – Guides decisions on packaging, storage, and distribution based on stability data. | 6. Not Universally Applicable – Some products may not require extensive Hold Time Study. |
| 7. Research and Development – Informs formulation development for enhanced stability and longevity. | 7. Variability in Results – Results can vary based on factors like batch composition and storage conditions. |
| 8. Supply Chain Management – Enables accurate projection of shelf life for better inventory management. | 8. Ethical Considerations – Longer hold times could lead to delays in delivering medications to patients. |
| 9. Market Competitiveness – Builds consumer trust by demonstrating product reliability and consistency. | 9. Impact on Innovation – Overly strict stability requirements might hinder the introduction of innovative products. |
| 10. Continuous Improvement – Supports ongoing process refinement and adaptation to industry changes. | 10. Balancing Act – Striking the right balance between stability and innovation can be challenging. |
| 11. Global Trade – Facilitates international trade with recognized stability data. | 11. Limited Long-Term Data – Extended studies may not reflect real-world conditions due to varying circumstances. |
| 12. Patient Safety – Ensures that pharmaceutical interventions remain safe and effective. | 12. Limited Short-Term Insights – Short hold time study might not capture longer-term stability issues. |
Frequently Asked Questions
What are Hold Time Studies in the context of the pharmaceutical industry?
Answer: Hold Time Study are controlled experiments conducted to assess the stability and integrity of pharmaceutical products over a designated period. These studies involve subjecting samples to various storage conditions to understand how they fare in terms of quality and efficacy over time.
Why are Hold Time Studies important in the pharmaceutical industry?
Answer: Hold Time Study are vital as they provide empirical data on the stability of pharmaceutical products. This data is essential for regulatory compliance, ensuring product quality, making informed decisions about shelf life and storage, and mitigating potential risks associated with degradation.
How do Hold Time Studies contribute to regulatory compliance?
Answer: Regulatory agencies require pharmaceutical companies to demonstrate the stability and quality of their products. Hold Time Study generate data that supports regulatory submissions, showing that products remain safe and effective over their intended shelf life.
What factors are controlled during Hold Time Studies?
Answer: Hold Time Study control variables such as temperature, humidity, and light, simulating real-world storage conditions. These controlled conditions help assess how products respond to environmental factors that could affect their stability.
What types of analytical techniques are used in Hold Time Studies?
Answer: Analytical techniques like spectroscopy, chromatography, and molecular assays are employed to evaluate samples during Hold Time Studies. These techniques provide insights into changes in potency, degradation, and other attributes.
How do Hold Time Studies impact manufacturing processes?
Answer: Hold Time Study help optimize manufacturing processes by determining the optimal hold time for products. This reduces waste, improves resource allocation, and enhances cost efficiency.
What benefits do Hold Time Studies offer in terms of patient safety?
Answer: Hold Time Studies ensure that pharmaceutical products maintain their efficacy and safety over their intended shelf life. This contributes to patient safety by delivering products that consistently meet quality standards.
Can Hold Time Studies influence research and development?
Answer: Yes, data from Hold Time Studies can guide research and development efforts. This information helps in formulating products that exhibit enhanced stability and longevity, leading to improved patient outcomes.
Are there any limitations to Hold Time Studies?
Answer: Hold Time Studies can be resource-intensive and time-consuming. Additionally, they might not capture all potential degradation mechanisms, and the interpretation of data can be complex due to various influencing factors.
How can pharmaceutical companies use the results of Hold Time Studies for decision-making?
Answer: Hold Time Study results can guide decisions on packaging, labeling, storage conditions, and even inform marketing strategies by demonstrating the stability and reliability of products.
What role do Hold Time Studies play in supply chain management?
Answer: Hold Time Studies help in accurately projecting a product’s shelf life, enabling effective supply chain management and preventing products from expiring before reaching end-users.
Can Hold Time Studies impact a pharmaceutical company’s market competitiveness?
Answer: Absolutely, Hold Time Studies contribute to building consumer trust by demonstrating the reliability and consistency of products. This can enhance the company’s reputation and competitiveness in the market.
How do Hold Time Studies address global trade requirements?
Answer: Hold Time Studies provide standardized stability data that regulatory bodies across different countries can recognize, facilitating international trade of pharmaceutical products.
What are some ethical considerations related to Hold Time Studies?
Answer: Longer hold times could potentially lead to delays in delivering medications to patients who need them. Striking a balance between stability testing and timely availability is essential.
How can Hold Time Studies impact the introduction of innovative products?
Answer: Strict stability requirements might hinder the timely introduction of innovative products. Striking a balance between stability and innovation is crucial for product development.
What are the implications of Hold Time Studies for patient well-being?
Answer: Hold Time Studies contribute to patient well-being by ensuring that pharmaceutical interventions remain safe and effective, providing confidence in the products they rely on for their health.
How does the interpretation of Hold Time Study results require expertise?
Answer: Interpreting Hold Time Study data demands a thorough understanding of analytical methods, statistical analysis, and the context in which the products will be used, ensuring accurate conclusions.
How do Hold Time Studies address variability in results?
Answer: Hold Time Studies consider potential variability by using statistically significant sample sizes and assessing data trends over time to account for potential deviations.
Can Hold Time Studies provide insights into both short-term and long-term product stability?
Answer: Yes, Hold Time Studies can offer insights into both short-term and long-term stability, helping manufacturers understand how their products will perform over varying timeframes.
How can companies strike a balance between the benefits and challenges of Hold Time Studies?
Answer: Companies can optimize Hold Time Studies by carefully designing experiments, utilizing advanced analytical techniques, and considering the specific needs of each product and market.
What is the overarching goal of Hold Time Studies in the pharmaceutical industry?
Answer: The main goal of Hold Time Studies is to ensure that pharmaceutical products maintain their efficacy, safety, and quality over time, benefiting both patients and the industry as a whole.


1 thought on “Hold Time Study in Pharma 2024”