Hydrophobic and hydrophilic filters are two distinct types of filters used in various industries and applications. These filters differ in their behavior towards water, making them suitable for specific filtration needs. In this article, we will explore the characteristics, advantages, and applications of hydrophobic and hydrophilic filters.
Hydrophobic Filters:
Hydrophobic filters repel water and are designed to allow the passage of non-aqueous substances while preventing the intrusion of water. They are typically made of hydrophobic polymers or fluorinated materials. The key features and applications of hydrophobic filters include:

- Water Repellency: Hydrophobic filters have a strong resistance to water intrusion due to their hydrophobic nature. They are ideal for applications where water exclusion is critical.
- Gas and Vapor Filtration: Hydrophobic filters are efficient in removing water droplets and aqueous phases from gas and vapor streams. They find applications in the oil and gas industry, laboratory settings, and vapor-phase filtration.
- Non-Aqueous Substances: These filters selectively filter non-aqueous substances while allowing the passage of gases or solvents. They are widely used in applications involving organic solvents or hydrocarbon streams.
- High-Temperature Resistance: Hydrophobic filters exhibit resistance to high temperatures, typically up to 100°C (212°F). This property makes them suitable for applications involving elevated temperatures.
Hydrophilic Filters:
Hydrophilic filters attract and retain water, allowing the passage of aqueous solutions while effectively filtering out particulates and microorganisms. They are typically composed of hydrophilic polymers or cellulose-based materials. The key features and applications of hydrophilic filters include:
- Water Attraction: Hydrophilic filters have an affinity for water molecules, facilitating the passage of aqueous solutions through the filter media. They are widely used in water and wastewater treatment, as well as in biomedical and pharmaceutical applications.
- Particle and Microorganism Removal: Hydrophilic filters are effective in separating suspended solids, colloidal particles, and microorganisms from liquids. They are crucial in ensuring the removal of impurities and contaminants.
- Compatibility with Aqueous Solutions: These filters are compatible with a wide range of aqueous solutions and biological fluids. They find applications in various fields, including biomedical research, pharmaceuticals, and food and beverage production.
- Sterile Filtration: Hydrophilic filters are commonly used for sterile filtration to ensure the removal of bacteria, viruses, and other microorganisms from liquids. They play a vital role in maintaining the sterility of pharmaceutical products and laboratory samples.
In summary, hydrophobic filters and hydrophilic filters serve distinct purposes in filtration processes. Hydrophobic filters repel water and are suitable for applications requiring the exclusion of water, while hydrophilic filters attract and retain water, making them ideal for filtering aqueous solutions and removing impurities. Understanding the characteristics and applications of these filters helps in selecting the appropriate filter type for specific filtration needs.

Hydrophilic filter vs Hydrophobic filters
| Hydrophilic Filters | Hydrophobic Filters |
|---|---|
| Affinity for water molecules | Resistance to water intrusion |
| Allow passage of water and aqueous solutions | Prevent water passage, enabling selective filtration of non-aqueous substances |
| Effective in separating suspended solids, colloidal particles, and microorganisms from liquids | Efficient in removing water droplets and aqueous phases from hydrocarbon streams |
| Widely used in biomedical and pharmaceutical applications for sterile filtration and sample preparation | Valuable in the oil and gas industry for gas purification, oil-water separation, and vapor-phase filtration |
| Suitable for water and wastewater treatment, ensuring the removal of impurities and contaminants | Find applications in solvent filtration, removing particulates, and ensuring clean samples in laboratory settings |
| High flow rates, low protein binding, and excellent chemical compatibility | Superior performance, including high dirt-holding capacity and resistance to wetting |
| Versatile, compatible with a wide range of liquids, including aqueous solutions and organic solvents | Versatile, accommodating various fluid types, from hydrocarbons to aggressive chemicals |
| Play a crucial role in environmental monitoring, aiding in the removal of pollutants from water samples | Contribute to clean air environments by selectively trapping organic vapors and odors |
| Tailorable pore sizes for precise control over filtration performance | Tailorable pore sizes for specific separation requirements |
| Important in food and beverage production, biotechnology, analytical laboratories, and more | Essential in the oil and gas industry, laboratory settings, environmental monitoring, and diverse applications |
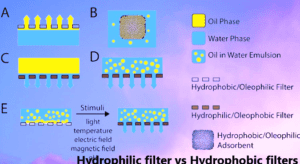
Filter integrity test for hydrophobic filters and Hydrophilic Filters
Hydrophobic filters play a crucial role in various applications, where they serve as barriers against water intrusion while allowing the passage of non-aqueous substances. To ensure their reliable performance, it is essential to conduct filter integrity tests that assess the integrity and efficiency of these filters. In this article, we delve into the filter integrity test for hydrophobic filters, exploring its significance, methodologies, and the insights it provides to ensure optimal filtration outcomes.
Significance of Filter Integrity Testing:
Filter integrity testing is a critical step in quality control and validation processes for hydrophobic filters. It verifies the filter’s structural integrity, confirms its ability to retain particles, and ensures that it maintains its hydrophobic barrier. By conducting filter integrity tests, manufacturers and users can have confidence in the filters’ performance and reliability, mitigating the risks of contamination or compromised filtration efficiency.
Common Filter Integrity Test Methods:
- Bubble Point Test: The bubble point test is a widely used method to assess the integrity of hydrophobic filters. It involves applying a gas or a liquid with low surface tension to the upstream side of the filter. Gradually increasing the pressure, the test determines the pressure at which gas or liquid starts to pass through the filter. This pressure, known as the bubble point, indicates the minimum pressure required to overcome the hydrophobic barrier and serves as a measure of the filter’s integrity.
- Diffusion Test: The diffusion test measures the filter’s ability to retain specific particles of a known size. In this test, a particle suspension is introduced to the upstream side of the filter, and the downstream effluent is collected and analyzed. By comparing the particle concentration in the upstream and downstream samples, the efficiency of particle retention can be determined. A high particle retention rate indicates good filter integrity.
- Integrity Test with Water Intrusion: In addition to the water intrusion test mentioned earlier, which assesses the hydrophobicity of filters, it can also be considered as an integrity test. By subjecting the filter to a specified pressure differential and monitoring for any water breakthrough, this test ensures that the filter maintains its hydrophobic barrier and does not allow water intrusion, which could compromise filtration efficiency or contaminate the filtrate.
Interpreting Filter Integrity Test Results:
The results of filter integrity tests provide crucial information about the filter’s performance. Passing the test with no detected breaches or significant particle passage indicates that the filter has maintained its structural integrity and hydrophobic barrier, ensuring reliable filtration. In case of failure, further investigation is required to identify the cause of the breach, such as defects, improper handling, or degradation, which can affect the filter’s performance.
Applications of hydrophobic and hydrophilic filters
| Application | Hydrophobic Filters | Hydrophilic Filters |
|---|---|---|
| Sterile Filtration | ✓ | ✓ |
| Drug Formulation and Delivery | ✓ | ✓ |
| Air and Gas Filtration | ✓ | ✓ |
| Bioburden Control | ✓ | ✓ |
| Removal of Particulates | ✓ | ✓ |
| Clarification and Prefiltration | ✓ | ✓ |
| Parenteral and Injectable Filtration | ✓ | ✓ |
| Buffer and Media Filtration | ✓ | |
| Virus Removal | ✓ | |
| Mycoplasma Filtration | ✓ | |
| Protein Purification and Concentration | ✓ | |
| Endotoxin Removal | ✓ |
Quality Assurance

Technical Details of Hydrophobic Filters
| Property | Description |
|---|---|
| Material Composition | The varied range available, from sub-micron to macro sizes. |
| Pore Size | The varied range is available, from sub-micron to macro sizes. |
| Filtration Mechanism | Based on size exclusion and hydrophobicity properties |
| Hydrophobic Coating | Optional surface treatment to enhance hydrophobicity. |
| Maximum Operating Temperature | Resistant to high temperatures, typically up to 100°C (212°F). |
| Compatibility | Compatible with a wide range of chemicals and solvents. |
| Flow Rates | High flow rates due to open structure and low resistance. |
| Sterility Assurance | Available in sterile formats for critical applications. |
| Bubble Point Pressure | Represents the minimum pressure required to overcome the hydrophobic barrier. |
| Particle Retention Efficiency | Efficient retention of particles based on the specified size. |
| Burst Pressure | Can withstand high-pressure differentials without failure. |
| Chemical Resistance | Resistant to chemical degradation and aggressive substances. |
| Application Range | Widely used in industries such as pharmaceuticals, biotechnology, and air/gas filtration. |
Technical Details of Hydrophilic Filters
| Property | Description |
|---|---|
| Material Composition | Typically made of hydrophilic polymers or cellulose-based materials |
| Pore Size | Varied range available, from sub-micron to macro sizes |
| Filtration Mechanism | Based on size exclusion and affinity for water molecules |
| Hydrophilic Coating | Optional surface treatment to enhance wettability and water permeability |
| Maximum Operating Temperature | Generally suitable for temperatures up to 60°C (140°F) |
| Compatibility | Compatible with a wide range of aqueous solutions and biological fluids |
| Flow Rates | Controlled flow rates to optimize filtration performance |
| Sterility Assurance | Available in sterile formats for critical applications |
| Bubble Point Pressure | Represents the minimum pressure required to wet the filter surface and initiate liquid flow |
| Particle Retention Efficiency | Efficient retention of particles based on specified size |
| Burst Pressure | Can withstand pressure differentials without failure |
| Chemical Resistance | Varies depending on the material composition |
| Application Range | Used in biomedical research, pharmaceuticals, food and beverage, and water filtration |
Frequently Asked Questions
What materials are commonly used in the construction of hydrophobic filters?
Answer: Hydrophobic filters are typically made of hydrophobic polymers or fluorinated materials.
How do hydrophobic filters repel water?

Answer: Hydrophobic filters repel water due to their surface properties, which prevent the intrusion of water molecules.
What are the primary applications of hydrophobic filters?
Answer: Hydrophobic filters are commonly used in gas and vapor filtration, oil and gas applications, and filtration of non-aqueous substances.
How do hydrophilic filters attract and retain water?
Answer: Hydrophilic filters have an affinity for water molecules, allowing them to attract and retain water while filtering out impurities.
What are the typical materials used in hydrophilic filters?
Answer: Hydrophilic filters are usually composed of hydrophilic polymers or cellulose-based materials.
What are some common applications of hydrophilic filters?
Answer: Hydrophilic filters are widely used in water and wastewater treatment, biomedical research, pharmaceuticals, and food and beverage production.
How do hydrophobic filters differ from hydrophilic filters in terms of filtration mechanism?
Answer: Hydrophobic filters rely on size exclusion and repelling water, while hydrophilic filters use both size exclusion and affinity for water molecules to separate particles and impurities.
What is the maximum operating temperature for hydrophobic filters?
Answer: Hydrophobic filters are typically resistant to high temperatures and can operate up to around 100°C (212°F).
Are hydrophobic filters compatible with organic solvents?
Answer: Yes, hydrophobic filters are compatible with organic solvents and hydrocarbon streams due to their selective filtration of non-aqueous substances.
What is the main purpose of using hydrophilic filters in sterile filtration?
Answer: Hydrophilic filters are commonly used in sterile filtration to remove bacteria, viruses, and other microorganisms from liquids, ensuring the sterility of pharmaceutical products and laboratory samples.
