An isolator is a highly controlled and enclosed system used in the pharmaceutical industry to provide a barrier between the operator and the product. It ensures the protection of the product from contamination and provides a controlled environment for pharmaceutical manufacturing, handling of potent compounds, and aseptic processes. Isolators are commonly used in sterile manufacturing, pharmaceutical compounding, and containment of hazardous materials. They offer superior product protection, operator safety, and environmental control, making them essential for maintaining the integrity and quality of pharmaceutical products.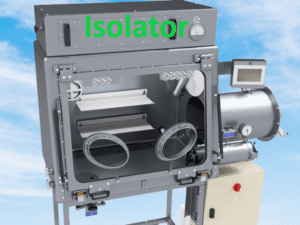
An isolator is a specialized enclosure or system used in various industries, including pharmaceuticals, to create a controlled environment that isolates a specific process or material. In the context of the pharmaceutical industry, isolators are primarily used for sterility testing, aseptic filling, and handling of potent or hazardous substances.
Isolators are designed to provide a high level of containment, preventing the entry of contaminants and protecting operators and the surrounding environment from exposure to potentially harmful substances. They consist of a sealed chamber or glovebox with integrated gloves or manipulators, allowing operators to perform tasks inside the isolator without direct contact with the contained materials.
The main purposes of using isolators in pharmaceutical applications are:
- Sterility Assurance: Isolators are commonly used in aseptic processing and sterile manufacturing to maintain a controlled environment and prevent contamination during critical processes, such as filling vials or preparing sterile compounds.
- Operator Safety: Isolators provide a physical barrier between operators and hazardous substances, such as potent drugs or toxic materials. This protects operators from exposure and reduces the risk of occupational hazards.
- Environmental Protection: Isolators help contain hazardous or infectious materials, preventing their release into the surrounding environment and minimizing the risk of cross-contamination.
- Product Integrity: By maintaining a controlled and clean environment, isolators help preserve the quality and integrity of pharmaceutical products, especially those sensitive to environmental factors like moisture, oxygen, or particulate matter.
Isolators often incorporate features such as HEPA (High-Efficiency Particulate Air) filters to maintain a sterile or clean environment, integrated decontamination systems for regular cleaning and disinfection, and pressure differentials to prevent the escape of contaminants.
The use of isolators in the pharmaceutical industry is subject to strict regulations and guidelines, including those related to good manufacturing practices (GMP) and the handling of hazardous substances. These regulations ensure that isolators are properly validated, maintained, and operated to meet the required standards of safety, quality, and efficacy in pharmaceutical manufacturing processes.
Working principle of Isolator in Pharmaceutical
The working principle of an isolator in the pharmaceutical industry is based on the concept of creating a controlled and isolated environment that provides a high level of containment and protection for both the product being processed and the operator.
Here are the key components and steps involved in the working principle of an isolator:
- Sealed Enclosure: The isolator consists of a sealed enclosure typically made of stainless steel or other suitable materials. It is designed to create a physical barrier that isolates the process or material inside from the external environment.
- Glove Ports/Manipulators: The isolator is equipped with glove ports or manipulators that allow operators to perform tasks inside the isolator without direct contact with the contained materials. These glove ports provide a means for operators to interact with the materials and equipment inside the isolator while maintaining a sealed environment.
- Air Handling System: The isolator is equipped with an air handling system that ensures a controlled environment inside the enclosure. This system typically includes HEPA (High-Efficiency Particulate Air) filters that remove airborne particles and microorganisms, maintaining a sterile or clean environment.
- Pressure Differential: The isolator maintains a controlled pressure differential between the inside and outside of the enclosure. This pressure differential helps prevent the escape of contaminants and ensures that any leaks occur from the outside to the inside, minimizing the risk of contamination.
- Decontamination System: Isolators often incorporate a decontamination system to facilitate regular cleaning and disinfection. This system may use methods such as vaporized hydrogen peroxide (VHP) or other suitable decontamination agents to sterilize the interior surfaces of the isolator.
- Monitoring and Control: Isolators are equipped with monitoring and control systems to ensure the integrity and safety of the process. These systems may include sensors for monitoring parameters such as pressure, temperature, humidity, and particle counts. They also enable the control of critical factors to maintain the desired conditions inside the isolator.
By combining these components and principles, isolators create a controlled environment that prevents the entry of contaminants, protects the product and operator, and ensures the integrity and quality of pharmaceutical processes. They are especially crucial for aseptic processing, containment of potent substances, and handling of sensitive or hazardous materials in the pharmaceutical industry.
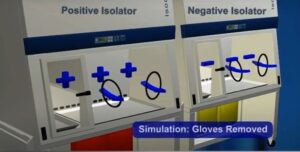
Positive and negative isolators:
Two different types of containment systems are used in the pharmaceutical industry. Here’s a brief explanation of positive and negative isolators:
Positive Isolators:
- Positive isolators maintain a higher pressure inside the isolator compared to the surrounding environment.
- The higher pressure inside the isolator prevents the entry of contaminants from the external environment into the isolator.
- Positive isolators are commonly used for aseptic processing, where maintaining a sterile environment is crucial.
- They provide a controlled and clean environment for processes such as an aseptic filling or sterility testing.
- Positive isolators are designed to protect the product from external contamination and maintain the sterility of the process.
Negative Isolators:
- Negative isolators maintain a lower pressure inside the isolator compared to the surrounding environment.
- The lower pressure inside the isolator ensures that any potential contaminants generated inside the isolator are contained within it.
- Negative isolators are commonly used for handling and containment of hazardous or potent substances.
- They provide a barrier to protect operators and the surrounding environment from exposure to these substances.
- Negative isolators are designed to prevent the release of hazardous materials or particles into the external environment.
Advantages and disadvantages of Pharmaceutical Isolators
| Advantages | Disadvantages |
|---|---|
| Provides a high level of containment | The initial cost can be higher compared to other solutions |
| Protects the product from contamination | Requires dedicated space and installation |
| Ensures operator safety | Limited mobility and flexibility |
| Creates a controlled and sterile environment | Regular maintenance and cleaning required |
| Enables aseptic processing | Training required for proper operation and maintenance |
| Reduces risk of cross-contamination | Requires validation and compliance with regulations |
| Maintains product integrity | May have limited visibility or access to the process |
| Helps prevent exposure to potent substances | Potential for equipment malfunction or downtime |
| Can be integrated with other pharmaceutical equipment |
Technical detail about Aseptic Isolators
- Application: Aseptic processing and sterile manufacturing
- Purpose: Provide a controlled and sterile environment
- Material: Typically made of stainless steel or other suitable materials
- Air Handling System: Incorporates HEPA filters for clean air supply
- Pressure Differential: Maintains positive pressure to prevent contamination
- Decontamination System: Utilizes methods like vaporized hydrogen peroxide (VHP)
- Glove Ports/Manipulators: Equipped with sealed glove ports or manipulators for operator interaction
- Monitoring and Control: Sensors monitor parameters like pressure, temperature, humidity, and particle counts
- Validation and Compliance: Requires validation to meet regulatory standards
- Operator Safety: Provides protection for operators from exposure to sterile products and contaminants
- Product Integrity: Preserves product quality and prevents cross-contamination
- Maintenance and Cleaning: Regular cleaning and maintenance are necessary
- Customization and Options: Can be customized to accommodate specific process requirements
- Integration with other equipment: Can be integrated with other pharmaceutical equipment
- Training and Documentation: Operator training and detailed documentation for operation and maintenance
This technical brief summarizes the key aspects of aseptic isolators. They are designed to create a controlled and sterile environment for aseptic processing and sterile manufacturing applications. Aseptic isolators utilize HEPA filters and pressure differentials to maintain cleanliness and prevent contamination. They incorporate decontamination systems, glove ports/manipulators, and monitoring sensors for operator safety, product integrity, and quality control. Compliance with validation, regular maintenance, and customization options are also important considerations.
Types of Pharmaceutical Isolator
There are several types of pharmaceutical isolators used in the industry, each designed to meet specific requirements and applications. The main types of pharmaceutical isolators include:
- Sterility Testing Isolators: These isolators are specifically designed for sterility testing of pharmaceutical products. They provide a controlled environment to prevent contamination during the testing process.
- Aseptic Filling Isolators: Aseptic filling isolators are used for aseptic processing and filling of sterile pharmaceutical products. They maintain a sterile environment and prevent contamination during the filling process.
- Containment Isolators: Containment isolators are used for handling and processing hazardous or potent compounds. They provide a high level of containment to protect operators and the surrounding environment from exposure to these substances.
- Biohazard Isolators: Biohazard isolators are designed for handling infectious materials, such as viruses or bacteria. They provide a barrier to protect operators and prevent the spread of pathogens.
- Animal Handling Isolators: These isolators are used in research or pharmaceutical facilities for handling laboratory animals. They provide a controlled environment to ensure the safety and well-being of the animals and prevent contamination.
- Glovebox Isolators: Glovebox isolators are a type of isolator that provides a sealed enclosure with integrated gloves or manipulators for operators to perform tasks inside the isolator without direct contact with the contained materials. They are commonly used for handling sensitive or hazardous substances.
- RABS (Restricted Access Barrier Systems): RABS are not technically isolators, but they are often categorized as such due to their similar purpose. RABS provides a barrier between the operator and the product but does not provide the same level of complete isolation as isolators. They are used in aseptic processing to minimize the risk of contamination.
These different types of pharmaceutical isolators serve specific functions and are designed to meet various industry requirements, ranging from sterility testing and aseptic filling to containment and handling of hazardous substances. The selection of the appropriate isolator depends on the specific application, the level of containment required, and regulatory guidelines.

Frequently Asked Questions:
What is a pharmaceutical isolator?
Answer: A pharmaceutical isolator is a specialized enclosure used in the pharmaceutical industry to provide a controlled and sterile environment for processes such as aseptic processing, containment of hazardous substances, or handling of sensitive materials.
What is the purpose of a pharmaceutical isolator?
Answer: The purpose of a pharmaceutical isolator is to prevent contamination, protect the product and operator, and maintain the integrity and quality of pharmaceutical processes.
What are the key components of a pharmaceutical isolator?
Answer: Key components of a pharmaceutical isolator include a sealed enclosure, glove ports or manipulators for operator interaction, an air handling system with HEPA filters, pressure differential control, decontamination systems, and monitoring and control systems.
What is the difference between positive and negative isolators?
Answer: Positive isolators maintain a higher pressure inside to prevent external contamination, while negative isolators maintain a lower pressure to contain hazardous materials generated inside.
How does a pharmaceutical isolator maintain sterility?
Answer: Pharmaceutical isolators maintain sterility by providing a controlled environment with filtered air, maintaining positive pressure, and incorporating decontamination systems to eliminate potential contaminants.
What are the applications of pharmaceutical isolators?
Answer: Pharmaceutical isolators are used for aseptic processing, sterile manufacturing, sterility testing, containment of hazardous substances, handling of sensitive materials, and animal handling in research or pharmaceutical facilities.
What are the advantages of using pharmaceutical isolators?
Answer: Advantages of pharmaceutical isolators include high containment levels, protection against contamination, operator safety, aseptic processing capability, reduced risk of cross-contamination, and product integrity preservation.
Are pharmaceutical isolators customizable?
Answer: Yes, pharmaceutical isolators can be customized to meet specific process requirements, such as size, layout, integration with other equipment, and additional features based on the application.
What are the regulatory requirements for pharmaceutical isolators?
Answer: Pharmaceutical isolators must comply with various regulatory guidelines, including Good Manufacturing Practices (GMP), specific industry standards, and validation requirements to ensure safety, quality, and compliance with regulations.
What maintenance is required for pharmaceutical isolators?
Answer: Regular maintenance and cleaning are necessary for pharmaceutical isolators to ensure optimal performance and prevent the buildup of contaminants. They require periodic validation and routine checks of components and systems for proper functioning.
