A sampling booth is a dedicated controlled environment in the pharmaceutical industry used for the safe and aseptic sampling of materials, products, and components. Discover how sampling booths provide a contamination-free space, prevent cross-contamination, and maintain sample integrity. Learn about their features, benefits, and applications in pharmaceutical manufacturing and quality control processes.
A sampling booth in the pharmaceutical industry is a specialized enclosure designed to provide a controlled and sterile environment for the sampling of pharmaceutical materials, products, and components. It ensures that the samples collected are free from contamination and maintains the integrity of the sampled substances.
Sampling booths are typically equipped with HEPA (High-Efficiency Particulate Air) filtration systems to create a clean, particle-free air environment. This prevents the introduction of contaminants during the sampling process, ensuring the accuracy and reliability of the collected samples.
The primary purpose of a sampling booth is to protect both the sampled materials and the operator from potential cross-contamination. By maintaining a controlled airflow pattern and physical separation, sampling booths minimize the risk of contamination between different samples and reduce the potential for exposure to hazardous or sensitive substances.
Use of Sampling booths in pharmaceutical processes.
- Raw Material Sampling: Pharmaceutical companies often need to sample incoming raw materials, such as active pharmaceutical ingredients (APIs), excipients, or packaging materials, to perform quality control testing and ensure compliance with specifications.
- Finished Product Sampling: Sampling booths are utilized for the collection of samples from finished pharmaceutical products, ensuring representative samples for quality analysis, stability testing, and batch release procedures.
- Environmental Monitoring: Sampling booths are also employed for environmental monitoring purposes, such as air or surface sampling to assess the cleanliness and sterility of manufacturing areas.
- Microbiological Sampling: In microbiology laboratories or sterile production areas, sampling booths provide a sterile environment for the aseptic collection of samples for microbial testing and analysis.

The design and features of sampling booths may vary depending on specific requirements, such as the type of samples, the level of cleanliness required, and regulatory guidelines. Some common features include a HEPA-filtered air supply, a work surface with smooth and cleanable materials, integrated lighting, and easy-to-use controls for maintaining the desired airflow and pressure differentials.
Overall, sampling booths play a critical role in maintaining the quality and safety of pharmaceutical products by providing a controlled environment for accurate and contamination-free sampling. They contribute to the adherence to regulatory requirements, product consistency, and the protection of both the samples and the personnel involved in the sampling process.
Technical Specification of sampling booth
| Technical Specification | Description |
|---|---|
| Purpose | Provides a controlled and sterile environment for sampling pharmaceutical materials |
| Airflow Control | Unidirectional airflow to maintain cleanliness and prevent cross-contamination |
| Airflow Velocity | Typically 0.45 to 0.65 m/s (90 to 130 fpm) |
| Filtration | HEPA (High-Efficiency Particulate Air) filters for air supply and exhaust |
| Cleanliness Standards | Compliance with relevant regulatory guidelines and cleanliness class requirements |
| Work Surface | Smooth, cleanable, and non-shedding material |
| Lighting | Integrated lighting system for proper visibility during sampling |
| Control System | User-friendly controls for adjusting airflow, pressure differentials, and alarms |
| Pressure Differentials | Maintains positive pressure inside the booth to prevent ingress of contaminants |
| Material Construction | Non-reactive, durable, and easy-to-clean materials |
| Noise Level | Low noise emissions for a comfortable working environment |
| Electrical Requirements | Complies with electrical safety standards and power supply requirements |
| Size and Configurations | Available in various sizes and configurations to accommodate different sampling needs |
| Validation and Testing | Regular validation and testing of airflow velocity, pressure differentials, and filters |
| Compliance | Conforms to relevant pharmaceutical and cleanroom standards and guidelines |
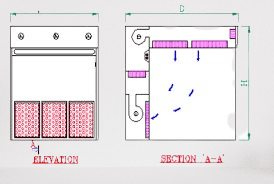
Guidelines to validate Sampling booth in Phama.
Validating a sampling booth in the pharmaceutical industry is crucial to ensure its performance, compliance with regulatory standards, and the integrity of the sampling process. Here are some guidelines for validating a sampling booth:
- Determine Validation Parameters: Identify the key parameters to be validated, such as airflow velocity, pressure differentials, particle count, and filter integrity. These parameters should align with regulatory requirements and industry guidelines.
- Develop a Validation Protocol: Create a detailed validation protocol that outlines the specific tests, procedures, and acceptance criteria for each parameter. The protocol should be comprehensive and include step-by-step instructions for the validation process.
- Perform Airflow Velocity Testing: Measure and verify the airflow velocity within the sampling booth using appropriate instruments. Ensure that the measured velocities are within the specified range to maintain the desired airflow pattern and prevent contamination.
- Conduct Pressure Differential Testing: Measure and monitor the pressure differentials between the sampling booth and its surroundings. Verify that the differential pressure is maintained within the recommended range to prevent the ingress of contaminants and ensure containment.
- Particle Count Analysis: Perform particle count tests to assess the cleanliness level inside the sampling booth. Compare the measured particle counts against the acceptable limits defined by regulatory guidelines and cleanliness standards.
- Filter Integrity Testing: Validate the integrity and efficiency of the HEPA filters used in the sampling booth. Conduct filter integrity tests, such as the DOP (Dispersed Oil Particulate) or PAO (Poly Alpha Olefin) tests, to ensure that the filters effectively capture and retain airborne particles.
- Evaluate Lighting and Work Surface: Assess the lighting levels and ensure proper illumination within the sampling booth. Verify that the work surface is clean, smooth, and suitable for the intended sampling activities.
- Perform Operational Qualification (OQ): Conduct OQ tests to verify the proper functioning of the sampling booth under normal operating conditions. This includes testing various operational parameters, alarm systems, and control functions.
- Document and Analyze Results: Record all the validation activities, test results, and observations in a comprehensive validation report. Analyze the results to determine whether the sampling booth meets the predetermined acceptance criteria.
- Regular Maintenance and Revalidation: Implement a maintenance plan to ensure ongoing performance and cleanliness of the sampling booth. Periodically revalidate the booth based on a predetermined schedule or when significant changes or modifications are made.
It is important to note that the validation process should align with regulatory requirements, industry guidelines, and internal quality management systems. Consult relevant regulatory authorities and refer to specific policies, such as those provided by the FDA, ISO, and local health authorities, for comprehensive validation requirements specific to your region.
Frequently Asked Questions:
What is the purpose of a sampling booth in pharmaceuticals?
Answer: The purpose of a sampling booth is to provide a controlled and sterile environment for the collection of samples in the pharmaceutical industry. It ensures that the samples collected are free from contamination and maintains the integrity of the sampled substances.
What is the recommended airflow velocity in a sampling booth?
Answer: The recommended airflow velocity in a sample booth is typically in the range of 0.45 to 0.65 meters per second (m/s) or 90 to 130 feet per minute (fpm). This velocity helps maintain a controlled airflow pattern and prevents the ingress of contaminants.
What type of filters are used in sampling booths?
Answer: Sample booths commonly use High-Efficiency Particulate Air (HEPA) filters for air supply and exhaust. These filters have high efficiency in capturing and removing airborne particles, ensuring a clean and sterile environment inside the booth.
How often should the HEPA filters in a sampling booth be replaced?
Answer: The frequency of filter replacement depends on factors such as the type and quantity of samples being collected, environmental conditions, and regulatory requirements. Generally, filters should be replaced according to the manufacturer’s recommendations or during regular maintenance schedules to maintain optimal performance.
What are the recommended cleanliness standards for a sampling booth?
Answer: The cleanliness standards for a sample booth depend on the specific application and regulatory guidelines. It is important to comply with relevant standards such as ISO 14644 for cleanroom classifications and the FDA’s Guidance for Industry on Sterile Drug Products.
How can the pressure differentials in a sampling booth be maintained?
Answer: Pressure differentials in a sample booth can be maintained by ensuring a positive pressure inside the booth relative to its surroundings. This can be achieved through proper airflow control and monitoring systems, such as adjusting the supply and exhaust air volumes.
Can a sampling booth be customized to meet specific requirements?
Answer: Yes, sample booths can be customized to meet specific requirements. They can be designed and configured to accommodate different sizes, layouts, and features such as glove ports, pass-through hatches, or additional monitoring systems based on the sampling needs and regulatory guidelines.
What are the validation requirements for a sampling booth?
Answer: The validation requirements for a sample booth include airflow velocity testing, pressure differential testing, particle count analysis, filter integrity testing, and evaluation of lighting and work surface cleanliness. The specific requirements may vary based on regulatory guidelines and industry standards.
How can the performance of a sampling booth be monitored and maintained?
Answer: The performance of a sample booth can be monitored and maintained through regular maintenance, periodic revalidation, and adherence to standard operating procedures. This includes proper filter maintenance, calibration of monitoring instruments and ensuring the cleanliness and integrity of the booth’s components.
Are there any specific regulations governing the use of sampling booths in pharmaceuticals?
Answer: Yes, the use of sample booths in pharmaceuticals is subject to regulatory requirements and guidelines. These regulations can vary depending on the region and country. It is important to consult relevant regulatory authorities, such as the FDA, EMA (European Medicines Agency), or local health authorities, for specific regulations and guidelines applicable to your jurisdiction.
