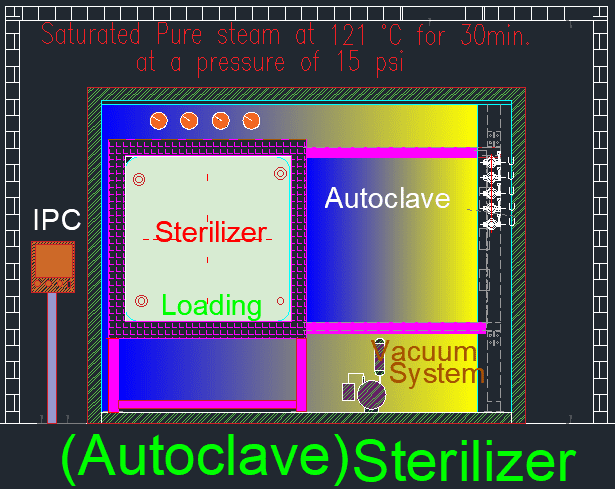
Sterilization is a cornerstone of various industries, especially in pharmaceutical and microbiological fields, where maintaining sterility of media and materials is vital for research, product development, and safety. Sterilization generally refers to the process of eliminating all forms of microbial life, including bacteria, viruses, spores, and fungi, to ensure that a medium or material is devoid of any viable microorganisms. While the standard sterilization temperature is 121°C, certain materials or culture media may require sterilization at lower temperatures to preserve their integrity. This leads to the introduction of Low-Temperature Sterilization (LTS), a process specially designed for heat-sensitive materials.

Standard Sterilization Process at 121°C :
Under normal circumstances, sterilization is performed at 121°C with a pressure of 15 psi (pounds per square inch) for 15 minutes. This is typically done in an autoclave, which uses steam under pressure to kill microorganisms. The high temperature and pressure ensure the complete destruction of all microbial life, including bacterial endospores, which are among the most resistant forms of life. This process is known as terminal sterilization and is employed for a variety of applications, such as preparing culture media, sterilizing equipment, and treating materials used in pharmaceutical manufacturing.
A crucial aspect of this process is the D-value, which refers to the time required to reduce the population of a specific microorganism by 90% (or one logarithmic cycle) at a given temperature. The effectiveness of sterilization can be calculated using this D-value to determine how long a material needs to be exposed to a particular temperature for effective sterilization.
However, while 121°C is effective for most materials, certain culture media and ingredients are sensitive to high temperatures, which can degrade their essential components. In these cases, Low-Temperature Sterilization becomes necessary.
Low-Temperature Sterilization (LTS) at 115°C :
Low-Temperature Sterilization is a specialized process used for sterilizing heat-sensitive materials, typically at a temperature of 115°C and a pressure of 10 psi. This method is commonly used for culture media like Reinforced Medium, Rappaport Vassiliadis Medium, Wilson and Blair’s BBS Agar, GN Broth, Triple Sugar Iron Agar, and Bismuth Sulphite Agar. These media contain heat-sensitive ingredients that degrade at higher temperatures, leading to the loss of their nutritive qualities, and thus, failing to support microbial growth effectively.
For instance, if these media are sterilized at 121°C, they may lose their essential properties, and while microorganisms may grow, they may not develop characteristic colonies. In simpler terms, the sterilized medium could still allow some form of microbial growth, but not in a way that is diagnostically useful due to the breakdown of critical nutrients during high-temperature exposure.
To compensate for the lower temperature used in LTS, the sterilization time must be increased to ensure the effective killing of microorganisms. A standard sterilization cycle at 115°C is run for 30 minutes, which is double the time of the 121°C process. Alternatively, the sterilization time can be calculated based on the D-value. If, for instance, the D-value of a microorganism at 115°C is 3.75 minutes, and an 8-log reduction (99.999999% reduction of the microbial population) is required, the sterilization time can be calculated as:Sterilization Time=D−value×Log Reduction=3.75 minutes×8=30 minutesSterilization\ Time = D-value \times Log\ Reduction = 3.75\ minutes \times 8 = 30\ minutesSterilization Time=D−value×Log Reduction=3.75 minutes×8=30 minutes
Comparison Between Standard Sterilization and Low-Temperature Sterilization :
| Aspect | Standard Sterilization Process | Low-Temperature Sterilization Process (LTS) |
|---|---|---|
| Temperature | 121°C | 115°C |
| Pressure | 15 psi (pounds per square inch) | 10 psi |
| Duration | 15 minutes | 30 minutes (or time calculated by D-value) |
| Purpose | Used for general sterilization of robust materials and culture media. | Designed for heat-sensitive materials and culture media. |
| Heat Sensitivity of Materials | Can degrade heat-sensitive components. | Prevents degradation of sensitive ingredients in media. |
| Media Sterilized | Suitable for most media and materials that can withstand high temperatures. | Used for media with heat-sensitive nutrients, e.g., Rappaport Vassiliadis Medium, Triple Sugar Iron Agar, GN Broth. |
| Effect on Microorganisms | Destroys all forms of microorganisms, including spores, effectively. | Effective in killing microorganisms but requires longer exposure due to lower temperature. |
| Characteristic Colony Formation | High temperatures may destroy some media components, preventing the growth of characteristic colonies. | Preserves the quality of the media, allowing for proper colony formation. |
| Biological Indicator Used for Validation | Bacillus stearothermophilus spores (ATCC 7953). | Bacillus subtilis spores (ATCC 5230). |
| Validation Process | Biological indicators are incubated at 55°C for 7 days. | Biological indicators are incubated at 35°C for 7 days. |
| Visual Indicator for Validation | Turbidity in biological indicators shows improper sterilization, while no turbidity indicates success. | Same as standard process; turbidity indicates failure, no turbidity means proper sterilization. |
| Applications | Suitable for sterilizing instruments, surgical materials, and standard culture media. | Essential for sterilizing temperature-sensitive media in microbiology and biotechnology. |
| Impact on Media Quality | May degrade sensitive components, affecting nutritive value and diagnostic capability of certain media. | Maintains the nutritive quality and diagnostic properties of heat-sensitive media. |
| Cycle Calculation (D-value) | Shorter D-values due to high temperature; D-value at 121°C is typically lower than at 115°C. | D-values are calculated based on 115°C; longer sterilization time required for desired log reduction. |
This table captures differences between the Standard Sterilization Process and Low-Temperature Sterilization, helping us to understand when to use each method based on the material and its sensitivity to heat.
Validation of Sterilization Processes :
Whether employing standard sterilization or low-temperature sterilization, validation is a critical part of ensuring the process is both effective and consistent. Sterilization validation involves ensuring that the sterilization cycle successfully kills all microbial life, particularly highly resistant bacterial spores.
The validation of both standard sterilization at 121°C and low-temperature sterilization (LTS) at 115°C involves careful evaluation, monitoring, and testing to ensure that the process consistently achieves the desired sterility assurance level (SAL). The differences in temperature and pressure between these methods require distinct validation protocols, particularly in cases involving heat-sensitive materials.

1. Overview of Sterilization Validation:
The validation process of any sterilization method is aimed at confirming that the sterilization cycle effectively eliminates viable microorganisms, including bacterial spores, which are the most resistant forms of life. This involves defining the parameters of the sterilization cycle, selecting appropriate biological indicators, and confirming the cycle’s reproducibility.
The three main stages in any sterilization validation are:
- Installation Qualification (IQ): Ensures that the sterilization equipment is installed according to specifications.
- Operational Qualification (OQ): Confirms that the equipment operates within its intended ranges, such as temperature and pressure.
- Performance Qualification (PQ): Demonstrates that the sterilization process consistently achieves the desired level of microbial kill in actual conditions.
2. Validation of Standard Sterilization Process (121°C):
Standard sterilization typically uses steam at 121°C and 15 psi for 15 minutes. This method is validated using biological indicators such as Bacillus stearothermophilus spores, known for their high resistance to heat. Validation involves testing for complete microbial inactivation without compromising the material’s properties.
Steps in Validation:
- Selection of Biological Indicator (BI): Bacillus stearothermophilus spores are used because they are highly resistant to moist heat, making them ideal for challenging the efficacy of the sterilization process.
- Load Testing: The sterilization process must be validated with representative loads to ensure that the entire load, regardless of its size or complexity, achieves sterility. Different load sizes and configurations are tested to determine the most challenging sterilization scenario.
- Incubation of Biological Indicators: After the sterilization cycle, BI strips or ampoules are incubated at 55°C for 7 days. The growth of the organism in the media indicates that the sterilization was insufficient. Turbidity or cloudiness in the medium signifies microbial growth, while no turbidity confirms that the sterilization process was successful.
- Validation of Sterilization Cycle: If the sterilization cycle repeatedly achieves complete microbial inactivation across different loads and configurations, the cycle is validated.
3. Validation of Low-Temperature Sterilization (115°C):
Low-temperature sterilization (LTS) at 115°C is necessary for materials or media that contain heat-sensitive ingredients. This method requires longer sterilization times due to the lower temperature and pressure used. The validation process for LTS is similar to that of standard sterilization, but there are key differences in terms of biological indicators and cycle time calculations.
Key Differences in LTS Validation:
- Biological Indicator (BI) for LTS: In LTS, Bacillus subtilis (also known as Bacillus atrophaeus) spores are used. These spores are more resistant to lower temperatures, which makes them suitable for validating the efficacy of LTS. Each strip contains approximately 1×10^6 spores, representing a high microbial load.
- Cycle Time Calculation: Due to the lower temperature, the sterilization cycle time must be increased. This is calculated using the D-value, which represents the time required to reduce the microbial population by 90%. For example, if the D-value at 115°C is 3.75 minutes, and an 8-log reduction in microbial population is required, the sterilization cycle must run for 30 minutes (3.75 x 8).
- Load Testing and Incubation: As with standard sterilization, representative loads are tested to ensure that the entire batch is adequately sterilized. BI strips and ampoules are incubated at 35°C for 7 days, and the process is validated if no microbial growth is detected.
4. Common Factors in Sterilization and LTS Validation:
Both standard sterilization and low-temperature sterilization follow similar validation principles, but the specific conditions differ based on the temperature and material sensitivity. The essential components of validation for both processes include:
- Biological Indicators: Critical to demonstrate microbial inactivation.
- Physical Parameters: Temperature, pressure, and time must be tightly controlled and monitored.
- Reproducibility: The sterilization cycle must consistently achieve the desired sterility assurance level across multiple runs.
- Documentation: All validation activities must be thoroughly documented, including biological indicator results, temperature, pressure data, and load configurations.
5. Challenges in Sterilization Validation:
Validating both standard and low-temperature sterilization can be challenging due to the following factors:
- Heat Sensitivity of Materials: In LTS, care must be taken to ensure that the sterilization process effectively eliminates microorganisms without degrading the heat-sensitive ingredients in the media.
- Cycle Optimization: Determining the optimal cycle time for each specific material or media requires careful testing and calculation based on the D-value.
- Consistency: Both processes must be validated to ensure they consistently achieve sterility across different load sizes, compositions, and environmental conditions.
Conclusion:
The validation of sterilization processes, whether at standard or low temperatures, is essential to ensure that the processes consistently deliver the required sterility assurance level without compromising the integrity of the materials being sterilized. The differences in temperature, cycle time, and biological indicators between standard sterilization at 121°C and low-temperature sterilization at 115°C necessitate tailored validation protocols. Through rigorous testing, monitoring, and documentation, both processes can be validated to guarantee safe and effective sterilization across diverse applications.
Frequently asked questions (FAQ):
1. What is the standard sterilization method in a hospital?
The standard sterilization method in hospitals is typically steam sterilization using an autoclave, which operates at 121°C for 15 minutes under 15 psi pressure. This ensures the elimination of all forms of microorganisms, including spores.
2. What is the ISO standard for sterilization?
The ISO standard for sterilization is ISO 11135 for ethylene oxide sterilization of medical devices and ISO 17665 for steam sterilization. These standards outline the requirements for validating and controlling sterilization processes.
3. What is the standard for autoclave sterilization?
The standard for autoclave sterilization follows ISO 17665, which specifies that materials must be sterilized at 121°C for a minimum of 15 minutes at 15 psi pressure.
4. What are the classifications of sterilization processes?
Sterilization processes can be classified into:
- Physical methods: Steam, dry heat, radiation.
- Chemical methods: Ethylene oxide, hydrogen peroxide, formaldehyde.
- Mechanical methods: Filtration.
- Plasma sterilization: Using ionized gas.
5. What is the standard sterile technique?
The standard sterile technique involves maintaining a sterile field to prevent contamination, ensuring all instruments, surfaces, and hands are sterilized. This technique includes using sterile gloves, gowns, masks, and sterile draping of patients.
6. What is the lowest level of decontamination?
The lowest level of decontamination is cleaning, which involves the removal of dirt and organic matter but does not necessarily kill all microorganisms.
7. What is the process of sterilization?
Sterilization is the process of killing or removing all forms of microbial life, including bacteria, viruses, fungi, and spores, from a surface, object, or fluid. Common methods include steam, dry heat, chemical agents, and radiation.
8. What is low-level disinfection?
Low-level disinfection refers to the elimination of most bacteria, some viruses, and some fungi but does not necessarily kill resistant organisms like bacterial spores.
9. What are the levels of sterilization?
Sterilization levels can be categorized as:
- Low-level disinfection: Eliminates most bacteria and viruses.
- Intermediate-level disinfection: Kills more resistant organisms.
- High-level disinfection: Kills all microorganisms except large numbers of spores.
- Sterilization: Completely eliminates all forms of microbial life.
10. What is the difference between high-level and low-level disinfectants?
- High-level disinfectants can kill most microorganisms, including resistant organisms like spores, with prolonged exposure.
- Low-level disinfectants are effective against most bacteria and some viruses but do not eliminate spores.
11. What is the standard sterilization method in a hospital?
The most widely used sterilization method in hospitals is steam sterilization via an autoclave, which operates at 121°C under pressure to eliminate all forms of microbial life.
12. What is the standard for sterilization validation?
The standard for sterilization validation is ISO 14937, which provides a framework for ensuring that a sterilization process achieves the required sterility assurance level (SAL) and is effective in all conditions.
13. What is the standard for sterility testing?
The standard for sterility testing is provided by ISO 11737, which specifies the tests needed to ensure that sterilized medical products are free from viable microorganisms.
14. What are the 7 methods of sterilization?
The 7 methods of sterilization are:
- Steam (Autoclaving)
- Dry Heat
- Ethylene Oxide Gas
- Hydrogen Peroxide Gas Plasma
- Radiation (Gamma or Electron Beam)
- Filtration
- Chemical Sterilization (e.g., Glutaraldehyde)
15. What is female sterilization called?
Female sterilization is commonly referred to as tubal ligation, a surgical procedure that blocks or cuts the fallopian tubes to prevent pregnancy.
16. What is the principle of sterilization?
The principle of sterilization is to eliminate or kill all forms of microbial life, including the most resistant spores, through physical, chemical, or mechanical means.
17. What is sterilization?
Sterilization is the process of making an object or environment free of all living microorganisms, including spores, through heat, chemicals, or radiation.
18. What is the classification of sterilization?
Sterilization can be classified into physical (e.g., heat, filtration), chemical (e.g., ethylene oxide), and radiation (e.g., gamma rays) methods, depending on the technique used.
19. What is the temperature for sterilization?
The common temperature for steam sterilization is 121°C, though it may be reduced to 115°C for low-temperature sterilization, and can go higher for certain dry heat sterilization processes.
20. What is the best sterilization process?
The best sterilization process depends on the material being sterilized, but steam sterilization (autoclaving) is widely regarded as the most reliable and effective for most applications.
21. Why 121 in autoclave?
The temperature 121°C is optimal for steam sterilization as it ensures the destruction of all microorganisms, including spores, while preventing excessive material degradation.
22. What is the timing of autoclave?
The standard time for autoclaving at 121°C is 15 minutes under 15 psi pressure, but this can vary depending on the load size and material.
23. What are the three processes of sterilization?
The three common processes of sterilization are:
- Steam (Autoclave)
- Dry Heat
- Chemical (e.g., Ethylene Oxide)
24. Which is a low to intermediate level disinfectant?
A quaternary ammonium compound (quats) is an example of a low to intermediate level disinfectant, effective against bacteria, fungi, and enveloped viruses.
25. What is the process of sterilization in CSSD?
In the Central Sterile Supply Department (CSSD), sterilization is typically done using steam autoclaves, with instruments first being cleaned, packed, and then subjected to high temperature and pressure.
26. What are the steps of the sterilization process?
The sterilization process involves:
- Cleaning: Removing debris.
- Packaging: Wrapping items for sterilization.
- Sterilizing: Applying heat, chemicals, or radiation.
- Monitoring: Ensuring the process is effective.
- Storage: Keeping items sterile until use.
27. What temperature is CSSD sterilization?
In CSSD, sterilization usually occurs at 121°C for 15 minutes under pressure using an autoclave, or at 134°C for faster cycles.
28. What is the full form of CSSD?
CSSD stands for Central Sterile Supply Department, responsible for cleaning, sterilizing, and distributing medical and surgical instruments.
29. What are the 7 methods of sterilization?
The 7 methods include steam, dry heat, ethylene oxide, hydrogen peroxide gas plasma, radiation, filtration, and chemical sterilization.
30. What are the standard conditions for sterilization?
The standard conditions for steam sterilization are 121°C temperature, 15 psi pressure, for 15 minutes.
31. What are the four types of sterilization?
The four types are:
- Heat Sterilization
- Chemical Sterilization
- Radiation Sterilization
- Mechanical Sterilization (e.g., filtration).
Guide lines of the Sterilization Validation: Ensuring Safety and Compliance