Sterilization in Place (SIP) is a crucial process employed in pharmaceutical plants to ensure the sterility of equipment and maintain aseptic manufacturing conditions. It offers several benefits and plays a vital role in pharmaceutical production.
Here are some key points regarding the implementation of SIP in pharmaceutical plants:
- Sterility Assurance:
- SIP is designed to achieve a high level of sterility by eliminating microorganisms from critical equipment and components used in the manufacturing process. It helps prevent contamination, ensuring the safety and efficacy of pharmaceutical products.
- Automation and Efficiency:
- SIP systems are automated, allowing for efficient and reliable sterilization without the need for manual disassembly or reassembly of equipment. This automation saves time, reduces labor costs, and minimizes the risk of human error or contamination.
- Equipment Integration:
- SIP systems are integrated into processing vessels, tanks, and other equipment used in pharmaceutical production. This integration enables sterilization to take place within closed systems, maintaining the integrity of the manufacturing environment.
- Sterilizing Agents:
- Various sterilizing agents can be used in SIP, depending on the equipment and process requirements. Steam, hot water, or chemical agents are commonly employed to achieve the desired sterilization conditions.
- Control Systems:
- SIP relies on advanced control systems that monitor and regulate temperature, pressure, and other critical parameters throughout the sterilization process. These systems ensure consistency, accuracy, and reproducibility of the sterilization cycles.
- Validation and Documentation:
- SIP processes require validation to demonstrate their effectiveness and compliance with regulatory standards. Validation studies and documentation are essential to ensure the reliability and traceability of the sterilization process.
- Regulatory Compliance:
- Implementing SIP in pharmaceutical plants helps meet regulatory requirements for sterility assurance. It ensures compliance with industry guidelines, such as Good Manufacturing Practices (GMP), which are vital for maintaining product quality and regulatory approval.
- Maintenance and Calibration:
- Regular maintenance and calibration of SIP systems and associated equipment are crucial to ensure their proper functioning and reliability. This helps prevent contamination and ensures the system’s performance adheres to established standards.
- Process Optimization:
- Continuous improvement and optimization of the SIP process are essential to enhance efficiency, reduce cycle time, and minimize resource consumption. Regular review and analysis of process data can identify opportunities for optimization and improved performance.
Key Considerations for Effective SIP
To ensure effective Sterilization-in-Place (SIP), there are several key considerations that should be taken into account. These considerations help optimize the SIP process and ensure the desired level of sterility. Here is a list of key considerations for effective SIP:
- Equipment Selection:
- Choose equipment that is suitable for SIP, considering factors such as material compatibility, design for effective distribution of the sterilizing agent, and accessibility for cleaning and monitoring.
- Process Assessment:
- Conduct a thorough assessment of the process requirements, including the type of microorganisms to be targeted, temperature and pressure tolerances, and regulatory guidelines.
- Sterilizing Agent Selection:
- Select an appropriate sterilizing agent, such as steam, hot water, or chemical agents, based on the equipment and process requirements. Consider the effectiveness against target microorganisms and compatibility with the equipment.
- Equipment Preparation:
- Ensure that the equipment is properly cleaned and free from visible residues or contaminants before initiating the SIP process. Validate cleaning procedures and consider the use of pre-rinse cycles to remove gross contamination.
- Validation and Documentation:
- Perform validation studies to confirm the effectiveness of the SIP process. Document all parameters, procedures, and results to ensure traceability and compliance with regulatory requirements.
- Control System Design:
- Implement a robust control system to monitor and control critical parameters, such as temperature, pressure, and exposure time. Ensure the control system is capable of providing real-time feedback and making necessary adjustments.
- Monitoring and Recording:
- Install sensors and monitoring devices to measure key parameters during the SIP process. Regularly monitor and record data to ensure consistency and identify any deviations that may require corrective action.
- Maintenance and Calibration:
- Establish a maintenance schedule to ensure the proper functioning of the SIP system and associated equipment. Calibrate sensors and instruments regularly to maintain their accuracy and reliability.
- Training and Personnel:
- Provide comprehensive training to personnel involved in the SIP process to ensure proper execution and adherence to established procedures. Emphasize the importance of following protocols, safety measures, and documentation requirements.
- Continuous Process Improvement:
- Continuously evaluate and optimize the SIP process. Analyze process data, identify areas for improvement, and implement corrective actions to enhance efficiency and effectiveness.
Sterilization by Pure Steam Generator from Pure Steam:
Sterilization in Place (SIP) is a crucial process employed in pharmaceutical plants to ensure the sterility of equipment and maintain aseptic manufacturing conditions. It offers several benefits and plays a vital role in pharmaceutical production. Here are some key points regarding the implementation of SIP in pharmaceutical plants:
- Sterility Assurance:
- SIP is designed to achieve a high level of sterility by eliminating microorganisms from critical equipment and components used in the manufacturing process. It helps prevent contamination, ensuring the safety and efficacy of pharmaceutical products.
- Automation and Efficiency:
- SIP systems are automated, allowing for efficient and reliable sterilization without the need for manual disassembly or reassembly of equipment. This automation saves time, reduces labor costs, and minimizes the risk of human error or contamination.
- Equipment Integration:
- SIP systems are integrated into processing vessels, tanks, and other equipment used in pharmaceutical production. This integration enables sterilization to take place within closed systems, maintaining the integrity of the manufacturing environment.
- Sterilizing Agents:
- Various sterilizing agents can be used in SIP, depending on the equipment and process requirements. Steam, hot water, or chemical agents are commonly employed to achieve the desired sterilization conditions.
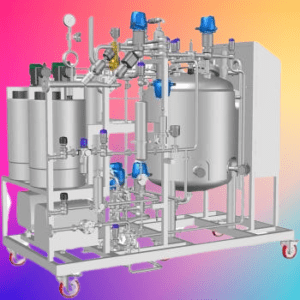
- Control Systems:
- SIP relies on advanced control systems that monitor and regulate temperature, pressure, and other critical parameters throughout the sterilization process. These systems ensure consistency, accuracy, and reproducibility of the sterilization cycles.
- Validation and Documentation:
- SIP processes require validation to demonstrate their effectiveness and compliance with regulatory standards. Validation studies and documentation are essential to ensure the reliability and traceability of the sterilization process.
- Regulatory Compliance:
- Implementing SIP in pharmaceutical plants helps meet regulatory requirements for sterility assurance. It ensures compliance with industry guidelines, such as Good Manufacturing Practices (GMP), which are vital for maintaining product quality and regulatory approval.
- Maintenance and Calibration:
- Regular maintenance and calibration of Sterilization-in-Place systems and associated equipment are crucial to ensure their proper functioning and reliability. This helps prevent contamination and ensures the system’s performance adheres to established standards.
- Process Optimization:
- Continuous improvement and optimization of the Sterilization-in-Place process are essential to enhance efficiency, reduce cycle time, and minimize resource consumption. Regular review and analysis of process data can identify opportunities for optimization and improved performance.
In summary, Sterilization-in-Place is an integral part of pharmaceutical plant operations, providing effective and reliable sterilization to maintain sterility and product integrity. The implementation of SIP systems, with their automation, documentation, and adherence to regulatory standards, ensures the highest levels of quality and safety in pharmaceutical manufacturing.
Advantages of Sterilization-in-Place (SIP) in Pharmaceutical:
| Advantages | Description |
|---|---|
| Effective Sterilization | Sterilization-in-Place ensures efficient and thorough sterilization, eliminating microorganisms from equipment and surfaces. |
| Time and Cost Savings | Sterilization-in-Place eliminates the need for disassembly and reassembly of equipment, saving time and reducing labor costs. |
| Reduced Risk of Contamination | By minimizing human intervention, Sterilization-in-Place reduces the risk of contamination associated with manual handling. |
| Enhanced Process Efficiency | Automated Sterilization-in-Place systems allow for optimized cleaning and sterilization cycles, improving overall efficiency. |
| Regulatory Compliance | SSterilization-in-Place tory requirements for sterility assurance, ensuring compliance with industry standards. |
| Preservation of Equipment Integrity | Sterilization-in-Place minimizes wear and tear on equipment by eliminating repetitive disassembly, preserving its longevity. |
| Consistency and Reliability | Automated control systems in Sterilization-in-Place ensure consistent and reliable sterilization parameters throughout the process. |
| Improved Safety | Sterilization-in-Place reduces the potential for accidents and injuries associated with manual handling of hot or hazardous materials. |
| Water and Resource Savings | Sterilization-in-Place systems often consume less water and cleaning agents compared to traditional cleaning methods. |
| Process Validation and Traceability | Sterilization-in-Place allows for easier process validation and traceability through documentation of cycle parameters and validation results. |
Frequently Asked Questions:
What is a SIP system in the pharmaceutical industry?
Answer: In the pharmaceutical industry, a Sterilization-in-Place system refers to a specialized setup that enables Sterilization in Place. It comprises integrated equipment and components designed to perform automated sterilization processes within processing vessels and systems without requiring disassembly.
How do you clean a SIP?
Answer: Cleaning a Sterilization-in-Place system involves several steps. First, the system is pre-rinsed to remove visible residues. Then, a cleaning solution, often a detergent or cleaning agent, is circulated throughout the system to remove contaminants. This is followed by rinsing with water to remove the cleaning solution, and finally, the system is drained and dried.
What is the temperature of clean-in-place (CIP)?
Answer: The temperature of clean-in-place (CIP) processes varies depending on the specific cleaning requirements and equipment being cleaned. Typically, CIP temperatures range from around 25°C (104°F) to 85°C (185°F).
What is the process of sterilization in place?
Answer: The process of Sterilization in Place (SIP) involves subjecting equipment or components to sterilizing conditions, such as steam or other sterilizing agents, within a closed system. The Sterilization-in-Place process utilizes predetermined temperature, pressure, and exposure time parameters to eliminate microorganisms and ensure sterilization.
What are the three types of sterilization?
Answer: The three types of sterilization commonly used are heat sterilization (such as autoclaving), chemical sterilization (using sterilizing agents or disinfectants), and radiation sterilization (gamma irradiation or electron beam).
What is the temperature for sterilization?
Answer: The temperature for sterilization varies depending on the sterilization method and the specific requirements of the materials being sterilized. In heat sterilization methods like autoclaving, temperatures typically range from 121°C (250°F) to 134°C (273°F) at high pressures.
What is the 6-step sterilization process?
Answer: The 6-step sterilization process commonly followed includes:
- Pre-cleaning: Removing visible residues or contaminants.
- Loading: Placing the items to be sterilized into the sterilization chamber or equipment.
- Air Removal: Removing air from the chamber to create a vacuum or controlled atmosphere.
- Sterilization: Applying the appropriate sterilizing agent, such as heat or chemicals, to eliminate microorganisms.
- Cooling: Allowing the items to cool down after sterilization to a safe handling temperature.
- Unloading: Retrieving the sterilized items from the chamber or equipment for further processing or use.
How does SIP contribute to regulatory compliance?
Answer: Sterilization-in-Place helps pharmaceutical manufacturers market regulatory requirements for sterility assurance, ensuring compliance with industry guidelines and standards such as Good Manufacturing Practices (GMP).
What are the key considerations for designing a SIP process?
Answer: Key considerations include equipment selection, process assessment, sterilizing agent selection, control system design, validation and documentation, and ongoing maintenance and calibration.
How is the effectiveness of SIP validated?
Answer: The effectiveness of Sterilization-in-Place is validated through studies that involve monitoring critical parameters, such as temperature, pressure, and exposure time, and using biological indicators to ensure microbial kill efficacy.