Analytical method validation, as guided by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), demands a nuanced understanding of the principles outlined in the document titled “Validation of Analytical Procedures: all the aspects and Methodology” are well explained in the below article, where precision and clarity, will embark on a comprehensive exploration of the characteristics, types, and methodologies crucial for successful analytical method validation.
Part I: Validation of Analytical Procedures – Definitions and Methodology
- Types of Analytical Procedures: Delving into the realm of validation, the document categorizes four common types of analytical procedures: Identification tests, Quantitative tests for impurities, Limit tests for the control of impurities, and Quantitative tests of the active moiety. While these serve as pillars, the document recognizes the potential inclusion of other procedures in subsequent editions.
- Objective of Validation: The primary objective of analytical procedure validation is to demonstrate its suitability for the intended purpose. A tabular summation outlines characteristics applicable to identification, control of impurities, and assay procedures. Accuracy, precision, specificity, detection limit, quantitation limit, linearity, and range emerge as pivotal validation characteristics.
- Changes and Revalidation: Acknowledging the dynamic nature of pharmaceutical development, the document underscores the need for revalidation in the face of changes such as alterations in drug substance synthesis, modifications in finished product composition, or adjustments to the analytical procedure itself.
Table: Characteristics Considered during Analytical Method Validation
| Type of Analytical Procedure | Identification | Testing for Impurities | Assay (Dissolution) | Assay (Content/Potency) |
|---|---|---|---|---|
| Accuracy | – | + | – | + |
| Precision | Repeatability | Intermediate Precision | – | – |
| Specificity (2) | + | + | + | + |
| Detection Limit | – | – (3) | + | – |
| Quantitation Limit | – | + | – | – |
| Linearity | – | + | – | + |
| Range | – | + | – | + |
- In cases where reproducibility has been performed, intermediate precision is not needed.
- Lack of specificity in one analytical procedure could be compensated by other supporting procedures.
- May be needed in some cases.
Glossary: The glossary elucidates crucial terms such as analytical procedure, specificity, accuracy, precision, repeatability, intermediate precision, reproducibility, detection limit, quantitation limit, linearity, range, and robustness.
Part II: Validation of Analytical Procedures – Methodology
- Introduction: This section serves as a complement to the parent guideline, providing guidance on considering validation characteristics for each analytical procedure. The complexity deepens as the document navigates through specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness.
- Specificity: An in-depth exploration of specificity investigates identification tests, determination of impurities, and assay procedures. The document highlights the need for a combination of procedures to achieve the necessary level of discrimination, especially in cases where complete discrimination is challenging.
- Linearity: The linear relationship is evaluated across the range of the analytical procedure. Visual inspection, statistical methods, and mathematical transformations play a role in establishing linearity. Notably, certain analytical procedures like immunoassays may require a different approach.
- Range: Derived from linearity studies, the specified range is pivotal and varies based on the intended application. Minimum specified ranges for assays, content uniformity, and dissolution testing are delineated, emphasizing the critical role each plays in the validation process.
- Accuracy: Accuracy assessment, integral to validation, involves several methods such as application to analytes of known purity, comparison with well-characterized procedures, and inference based on established precision, linearity, and specificity.
- Precision: Validation of precision encompasses repeatability, intermediate precision, and reproducibility. The document recommends an experimental design matrix to study variations in days, analysts, equipment, etc., emphasizing the need to understand the effects of random events on precision.
- Detection Limit: Determining the detection limit involves visual evaluation, signal-to-noise ratio, and the standard deviation of the response and slope. Different approaches cater to both non-instrumental and instrumental methods, ensuring a comprehensive understanding of the analyte’s detectability.
- Quantitation Limit: Similar to the detection limit, establishing the quantitation limit involves visual evaluation, signal-to-noise ratio, and the standard deviation of the response and slope. With a focus on accuracy and precision, this parameter ensures reliable quantification of analytes.
- Robustness: Robustness evaluation, integral during the development phase, assesses the reliability of an analysis amid deliberate variations in method parameters. Factors such as stability of solutions and variations in chromatographic conditions are scrutinized, ensuring the analytical procedure’s resilience.
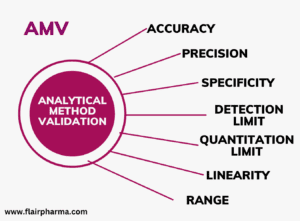
Key characteristics considered during analytical method validation:
| Characteristic | Description |
|---|---|
| Accuracy | The closeness of test results to the true or accepted reference value, indicating measurement correctness. |
| Precision | The degree of agreement between repeated measurements, showcasing the method’s consistency and reproducibility. |
| Specificity | The ability of the method to accurately measure the analyte in the presence of potential interfering substances, ensuring selectivity. |
| Sensitivity | The method’s capacity to detect small changes in analyte concentration, often linked to the limit of detection and quantification. |
| Linearity | The range over which the method demonstrates a proportional relationship between analyte concentration and response, ensuring accuracy. |
| Range | The span of concentrations or amounts for which the method is proven to be accurate, precise, and linear. |
| Robustness | The method’s resilience to variations in experimental conditions, demonstrating its reliability in real-world scenarios. |
| Ruggedness (or Intermediate Precision) | The reproducibility of results across different laboratories, instruments, operators, or other variable conditions. |
| Limit of Detection (LOD) | The lowest analyte concentration that can be reliably detected but not necessarily quantified, signaling sensitivity. |
| Limit of Quantification (LOQ) | The lowest analyte concentration that can be accurately and precisely measured, defining the method’s lower limit of usefulness. |
- Accuracy:
- Description: Accuracy reflects how close the test results are to the true or accepted reference value. It is a measure of the correctness of the measurements.
- Significance: Accurate results are crucial for ensuring that the method provides reliable information about the analyte being measured.
- Precision:
- Description: Precision measures the degree of agreement between repeated measurements. It showcases the method’s consistency and reproducibility.
- Significance: A precise method consistently produces similar results under the same conditions, indicating reliability in measurement.
- Specificity:
- Description: Specificity refers to the ability of the method to accurately measure the analyte in the presence of potential interfering substances. It ensures selectivity for the analyte of interest.
- Significance: A specific method provides confidence that the measured signal is solely attributed to the analyte, preventing interference from other components.
- Sensitivity:
- Description: Sensitivity is the method’s capacity to detect small changes in analyte concentration. It is often linked to the limit of detection and quantification.
- Significance: A sensitive method can detect low concentrations of the analyte, enhancing the ability to study substances present in trace amounts.
- Linearity:
- Description: Linearity refers to the range over which the method demonstrates a proportional relationship between analyte concentration and response. It ensures accuracy across a spectrum of concentrations.
- Significance: A linear method allows for reliable quantification across a broad range of analyte concentrations, enhancing versatility.
- Range:
- Description: The range is the span of concentrations or amounts for which the method is proven to be accurate, precise, and linear.
- Significance: A well-defined range ensures that the method is suitable for the intended application and provides accurate results within specified limits.
- Robustness:
- Description: Robustness is the resilience of the method to variations in experimental conditions. It demonstrates the reliability of the method in real-world scenarios.
- Significance: A robust method maintains its performance despite minor variations in factors such as temperature, pH, and equipment, ensuring consistency.
- Ruggedness (or Intermediate Precision):
- Description: Ruggedness assesses the reproducibility of results across different laboratories, instruments, operators, or other variable conditions.
- Significance: A rugged method demonstrates that it can consistently produce reliable results in diverse settings, contributing to method transferability.
- Limit of Detection (LOD):
- Description: LOD is the lowest analyte concentration that can be reliably detected but not necessarily quantified, signaling the sensitivity of the method.
- Significance: A low LOD indicates the method’s ability to detect trace amounts of the analyte, making it suitable for applications requiring high sensitivity.
- Limit of Quantification (LOQ):
- Description: LOQ is the lowest analyte concentration that can be accurately and precisely measured, defining the method’s lower limit of usefulness.
- Significance: The LOQ sets the threshold for reliable quantification, ensuring that the method provides accurate results within a defined concentration range.
Factors that can have an effect on the robustness of an analytical method:
| Factor | Description |
|---|---|
| Column Variability | Variations in column performance, such as different lots or brands, can impact method robustness. |
| Mobile Phase Composition | Changes in the composition of the mobile phase, including variations in solvent proportions or pH, may affect method performance. |
| Temperature | Fluctuations in temperature, both of the environment and analytical instruments, can influence results. |
| Flow Rate | Variations in the rate at which the mobile phase travels through the system can impact method robustness. |
| Injection Volume | Changes in the volume of sample injected may affect peak resolution and overall method performance. |
| pH of the Mobile Phase | Alterations in the acidity or alkalinity of the mobile phase can influence the separation of analytes. |
| Column Oven Temperature | Adjustments to the column oven temperature can impact the efficiency and selectivity of the method. |
| Sample Matrix | Differences in the matrix of samples, such as varying matrices for real-world samples, can affect robustness. |
| Sample Storage Conditions | Variations in the conditions under which samples are stored may influence the stability of analytes. |
| Operator Technique | Variances in the way different operators execute the method can impact results, highlighting method robustness. |
| Instrumental Variability | Differences in instrument performance, calibration, or maintenance can affect the robustness of the method. |
| Wavelength (for UV detection) | Changes in the selected wavelength for UV detection in spectrophotometry can influence method robustness. |
Advantages or Benefits of method validation (AMV)
| AMV | Description |
|---|---|
| 1. Assurance of Accuracy | Method validation ensures that analytical methods provide accurate and reliable results by confirming the closeness of measured values to true values. |
| 2. Confidence in Results | Validated methods instill confidence in laboratory results, enhancing the reliability of data generated for product quality control, research, and regulatory compliance. |
| 3. Regulatory Compliance | Validated methods align with regulatory requirements, meeting the standards set by authorities such as the FDA, EMA, and other regulatory bodies, ensuring compliance with industry regulations. |
| 4. Improved Precision | Through validation, the precision of analytical methods is evaluated, leading to improved consistency and repeatability of results. This is critical for ensuring the reliability of data over time. |
| 5. Robustness Assessment | Method validation includes robustness testing, assessing the method’s resistance to small variations in parameters. Robust methods are less susceptible to minor changes, providing stability in routine use. |
| 6. Identification of Limitations | Validation helps identify the limitations of analytical methods, including factors that may impact performance. Understanding these limitations enables informed decision-making and appropriate method application. |
| 7. Facilitates Method Transfer | Validated methods are more easily transferable between laboratories, ensuring consistent results across different locations. This is particularly valuable in a globalized pharmaceutical or research environment. |
| 8. Increased Product Quality | By employing validated methods for quality control, pharmaceutical companies can enhance the quality of their products, meeting specifications and delivering safe and effective medications to consumers. |
| 9. Cost Savings | Effective method validation reduces the likelihood of errors and rework, leading to cost savings in terms of time, resources, and materials. The investment in validation pays off through increased efficiency. |
| 10. Continuous Improvement | The validation process encourages a culture of continuous improvement in analytical processes. Regular review and revalidation lead to updates and enhancements, keeping methods in line with evolving industry standards. |
Example of the analytical method validation (AMV)
example of the analytical method validation for the assay determination of a specific active pharmaceutical ingredient (API) in a pharmaceutical plant laboratory. The method used for this example is high-performance liquid chromatography (HPLC).
Analytical Method: HPLC Assay for API
Objective: To validate the HPLC method for the accurate quantification of a specific API in the pharmaceutical product.
Validation Characteristics:
- Accuracy:
- Procedure: Spike known amounts of the API into a placebo sample.
- Analysis: Perform the HPLC assay on spiked samples.
- Criteria: The recovery should be within 98-102%.
- Precision:
- Repeatability: Analyze six replicate samples of the same concentration on the same day.
- Intermediate Precision: Analyze the same samples on three different days by different analysts.
- Criteria: Relative standard deviation (RSD) should be ≤ 2%.
- Linearity:
- Procedure: Prepare standard solutions at different concentrations covering the expected range.
- Analysis: Analyze each standard solution by HPLC.
- Criteria: A linear relationship between concentration and response, with a correlation coefficient (r) ≥ 0.999.
- Range:
- Procedure: Analyze samples at the lower and upper limits of the expected concentration range.
- Criteria: The method should provide accurate and precise results across the specified range.
- Specificity:
- Procedure: Analyze the API in the presence of potential impurities, degradants, and excipients.
- Criteria: The API peak should be well-resolved from other components, demonstrating specificity.
- Detection Limit (DL) and Quantitation Limit (QL):
- Procedure: Determine DL and QL based on signal-to-noise ratios or standard deviation methods.
- Criteria: DL and QL should be established where the API can be reliably detected and quantified.
- Robustness:
- Procedure: Introduce deliberate variations in method parameters (e.g., mobile phase composition, flow rate).
- Analysis: Assess the impact on method performance.
- Criteria: The method should remain robust under small variations.
Documentation:
- Validation Protocol: A detailed document outlining the validation plan, including acceptance criteria.
- Validation Report: A comprehensive report summarizing the results of each validation characteristic.
- Standard Operating Procedure (SOP): An updated SOP for the validated HPLC method.
Conclusion: The HPLC assay method for the API is validated, demonstrating accuracy, precision, linearity, specificity, and robustness. The results meet predefined acceptance criteria, ensuring the reliability of the method for routine use in the pharmaceutical plant laboratory.
Frequently asked Questions about AMV
What is Analytical Method Validation?
Analytical Method Validation (AMV) is a systematic process used to confirm that an analytical method is suitable for its intended purpose. It involves demonstrating that the method is accurate, precise, specific, robust, and capable of providing reliable and consistent results.
What is the Need for Analytical Method Validation?
The need for AMV arises to ensure the reliability and accuracy of analytical results. Validation confirms that an analytical method is suitable for its intended application, preventing inaccurate data that could lead to incorrect conclusions, compromised product quality, or potential harm to patients in pharmaceutical contexts.
Why is it Essential to Adopt Validated Analysis Methods?
Adopting validated analysis methods is essential to ensure the accuracy, reliability, and reproducibility of analytical data. Validated methods provide confidence in the results, supporting critical decision-making processes in research, manufacturing, and quality control.
What is GMP Validation of Analytical Methods?
GMP (Good Manufacturing Practice) validation of analytical methods ensures that methods used in pharmaceutical manufacturing comply with regulatory requirements. It involves verifying that analytical methods are suitable for their intended use, following established guidelines to meet quality standards.
What is the RSD Limit for Analytical Method Validation?
The Relative Standard Deviation (RSD) limit for analytical method validation depends on the specific method, industry standards, and regulatory requirements. Typically, a lower RSD indicates higher precision, and acceptable limits vary based on the context and analytical technique.
What is Analytical Method Validation PDF?
An Analytical Method Validation PDF refers to a document or guideline in Portable Document Format (PDF) that provides information on the principles, procedures, and requirements for validating analytical methods. These documents often include industry-specific guidelines or regulatory standards.
What is Analytic Validation?
Analytic validation is the process of confirming that an analytical method is accurate, reliable, and suitable for its intended purpose. It encompasses assessing various parameters, such as precision, accuracy, specificity, and robustness, to ensure the method’s validity.
What is the RSD Limit for Impurities?
The RSD limit for impurities depends on the specific industry and regulatory requirements. It is essential to establish acceptance criteria based on the characteristics of the impurities and the analytical method used for their determination.
What is LOD and LOQ in Method Validation?
LOD (Limit of Detection) and LOQ (Limit of Quantification) are critical parameters in method validation. LOD is the lowest concentration of an analyte that can be reliably detected but not necessarily quantified. LOQ is the lowest concentration that can be quantified with acceptable precision and accuracy.
What are the 4 Validation Types?
The four common types of validation are:
- Prospective Validation: Conducted before routine use of the method.
- Concurrent Validation: Involves testing samples concurrently with routine use.
- Retrospective Validation: Involves analyzing historical data to validate a method.
- Revalidation: Conducted when changes are made to the method or process.
What is Analytical Method Validation in QA?
Analytical Method Validation in Quality Assurance (QA) involves ensuring that analytical methods used for testing meet predefined quality standards. QA oversees the validation process to guarantee the reliability and accuracy of analytical data in AMV.
What RSD is Acceptable?
The acceptable RSD (Relative Standard Deviation) varies depending on the industry, analytical method, and regulatory requirements. It is crucial to establish acceptance criteria based on the specific context and precision requirements of the analysis in the Analytical Method Validation .
What is the RSD Limit for HPLC?
The RSD limit for High-Performance Liquid Chromatography (HPLC) depends on factors such as the analyte, industry standards, and regulatory guidelines. Typically, a lower RSD indicates higher precision, and the acceptable limit varies based on specific requirements.
How Do You Calculate Impurity Level?
Impurity level can be calculated as a percentage using the formula: Impurity Level (%)=(Amount of ImpurityTotal Amount of Sample)×100Impurity Level (%)=(Total Amount of SampleAmount of Impurity)×100
This formula expresses the proportion of the impurity relative to the total amount of the sample, providing a percentage representation of the impurity level in the Analytical Method Validation .
What is AMV?
Answer: Analytical Method Validation is the process of demonstrating that an analytical method is suitable for its intended purpose by providing scientific evidence that it is reliable, reproducible, and capable of generating accurate and precise results.
Why is Analytical Method Validation important in the pharmaceutical industry?
Answer: Analytical Method Validation is critical in the pharmaceutical industry to ensure the accuracy and reliability of analytical methods used for testing drug substances and products. It helps in meeting regulatory requirements, ensuring patient safety, and maintaining the quality and efficacy of pharmaceutical products.
What are the key parameters assessed during Analytical Method Validation?
Answer: Key parameters assessed during Analytical Method Validation include accuracy, precision, specificity, linearity, range, detection limit, quantitation limit, and robustness. These parameters collectively ensure the method’s suitability for its intended purpose.
What is the difference between accuracy and precision in the context of Analytical Method Validation?
Answer: Accuracy refers to how close the measured values are to the true values, while precision is the degree of repeatability or reproducibility of the analytical method. Accuracy is assessed by recovery studies, while precision is evaluated through repeatability and intermediate precision studies.
How is linearity assessed during Analytical Method Validation?
Answer: Linearity is assessed by analyzing a series of standards at different concentrations and plotting the calibration curve. The correlation coefficient (R2) is used to evaluate how well the method’s response is linear over the specified range.
What is the purpose of specificity in Analytical Method Validation?
Answer: Specificity ensures that the analytical method accurately measures the analyte of interest without interference from other components. It involves evaluating potential sources of interference and demonstrating the method’s selectivity for the analyte.
How is robustness evaluated during Analytical Method Validation?
Answer: Robustness is assessed by intentionally varying method parameters within defined limits and evaluating the impact on results. It demonstrates the method’s capacity to remain unaffected by small variations in conditions, providing insight into its reliability.
What are the regulatory guidelines governing Analytical Method Validation?
Answer: Regulatory guidelines for Analytical Method Validation include those from organizations such as the International Council for Harmonisation (ICH), the United States Pharmacopeia (USP), and the European Medicines Agency (EMA). These guidelines provide industry standards and expectations for method validation.
How often should Analytical Method Validation be performed?
Answer: Analytical Method Validation should be performed during the method development phase and whenever there are significant changes to the method or its conditions. Routine revalidation may be necessary at defined intervals to ensure ongoing method performance.
What is the role of Analytical Method Validation in Good Manufacturing Practice (GMP)?
Answer: Analytical Method Validation is a fundamental aspect of GMP, ensuring that analytical methods used in the testing of pharmaceutical products meet quality standards. It contributes to the reliability and consistency of product quality, aligning with GMP principles for ensuring patient safety and product efficacy.