Corrective Action and Preventive Action (CAPA) in the pharmaceutical industry, including their key steps, benefits, and importance in ensuring product quality, patient safety, and regulatory compliance. Understand how CAPA processes help identify and address root causes of quality issues, promote continuous improvement, and mitigate risks to enhance pharmaceutical operations.
- CAPA is an aid for improving quality.
- CAPA is a means to detect recurring problems.
- CAPA is a management tool (a problem costs time and money, rejection, standstill, production delays, etc.).
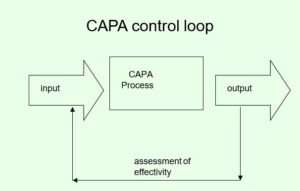
- CAPA is a closed system (control loop), driven by data.
- CAPA is also regarded as a subsystem (combination of systems).
Purpose of CAPA
A system that collects and analyses information, identifies and investigates product and quality aspects, takes measures to prevent the (repetition of) problems and verifies their effectivity.
- Corrective Action
- Immediate steps to repair damage
- symptom elimination
- emergency measures/firefighting
- Preventative Action
- Long-term steps to avoid repetition
- System improvement
- actions based on thorough root cause analysis
- Correction
- Immediate steps to repair damage
- symptom elimination
- emergency measures/firefighting
- Corrective Action
- Long-term steps to avoid repetition.
- System improvement.
- actions based on thorough root cause analysis.
- Preventative Action
- Avoid comparable situations (do an Assessment).
- This might result from planned, routine, or specific Risk Analysis studies.
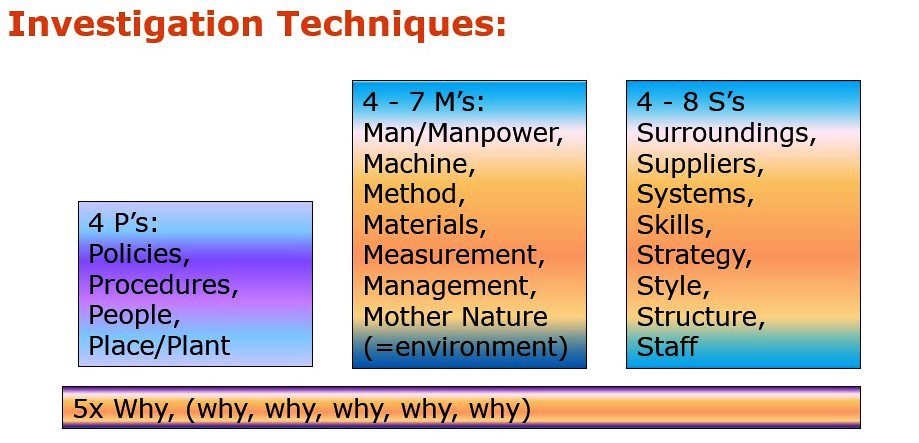
CAPA: Nine Steps
- Identify source data.
- Analyse source data and determine scope.
- Conduct an investigation, and search for the cause.
- Analyze the potential problem or the cause.
- Draw up an action plan.
- Evaluate and approve the plan (involve Management).
- Implement the plan.
- Monitor introduction/implementation.
- Assess effectivity.
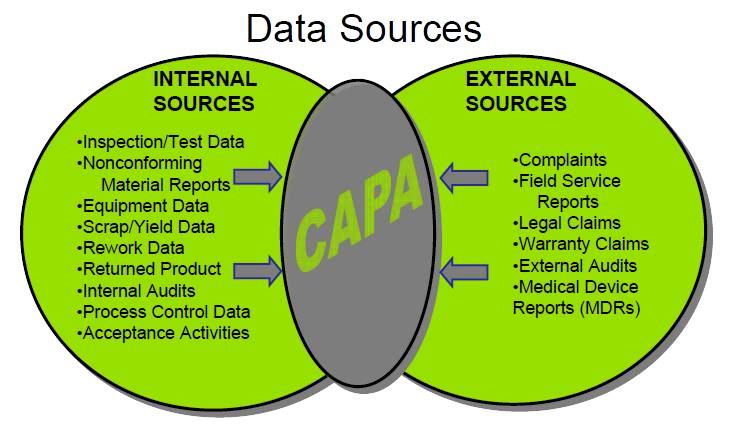
Step 1: Sources of CAPA input
Internal sources
- Deviations (planned and unplanned – non-conformances).
- Out of Specification (OOS), Out of (action) limits (OOL).
- Out of (calibration) tolerance (OOT).
- Internal audit observations, quality problems.
- Maintenance data (preventative or repair)
- Risk analysis, Error analysis.
- Trends.
- Process parameters, reprocessing.
- Product Quality Review.
- Change Control.
External sources:
- Complaints
- Recall
- External audit observations
- Returned products
- Supplier information
- (changes in) Regulations
Step 2: Source data analysis
- Clearly define the (potential) problem.
- Determine the (possible) scope of the problem.
- Estimate the (potential) impact and consequences of the problem with respect to, for example, quality, safety, and efficacy.
- Set priorities.
- Determine the need for any immediate actions/ corrections/ adjustments.
- Take immediate action to prevent further problems and minimize the impact/damage caused by the problem as far as possible – place suspect batches in quarantine.
Step 3: Investigation and Problem Analysis
- Determine the severity of the situation, conduct a risk analysis, and specify the depth of the investigation.
- Investigation system:
Formal or informal process, person/group, Herringbone model, checklist, investigation team, creativity (brainstorming), interviews, collect data. - Keep asking yourself “Why?”.
- A written investigation plan assures completeness.
- Distribution of tasks during the investigation; various disciplines (SMEs).
- Do not stop after finding one possible cause.

Step 4: Prepare an action plan
- If possible, apply the SMART method = Specific, Measurable, Attainable, Result-focused, Time.
- Determine the processes, procedures, and systems to be changed.
- The analysis should allow the best method for correcting the situation to be determined.
- Define clear objectives, milestones, and deliverables.
- Specify factors of success and effectivity assessment criteria.
- Describe the changes sufficiently to support understanding and assess the anticipated degree of success.
- Ideally, a plan should consist of the following phases:
- Preparation.
- Design.
- Implementation.
- Validation & Verification.
- Allocate tasks and responsibilities.
- Do not neglect the training aspect.
- Constantly ask yourself what you wish to achieve; when are you satisfied with the result?
- Be aware: Retraining the operator is not the ultimate CA.
Step 5: Management Approval
- If a plan is not granted approval, note the reason for rejection and suggestions for making the plan acceptable.
Step 6: Implementation
- Install a Project team (for large-scale CAPA plans).
- Use existing quality systems such as Change Control.
- Ensure adequately detailed descriptions of activities/tasks.
- Provide sufficient resources.
- Be conscious of a comprehensive and timely approach.
Step 7: Monitoring
- Written report.
- Tracking and tracing.
- Progress reports/management quality review.
- Training of the new situation.
Step 8: Check effectivity
- Often ignored and/or underestimated.
- True completion of the CAPA process must be found through evaluating all actions, including investigating whether the actions have not had an adverse effect on the product or service, for example.
- Usually QA responsibility.
- Work where possible with performance indicators.
- Devote attention to CAPA with internal audits.
- Proper audit trail of all decisions/actions.
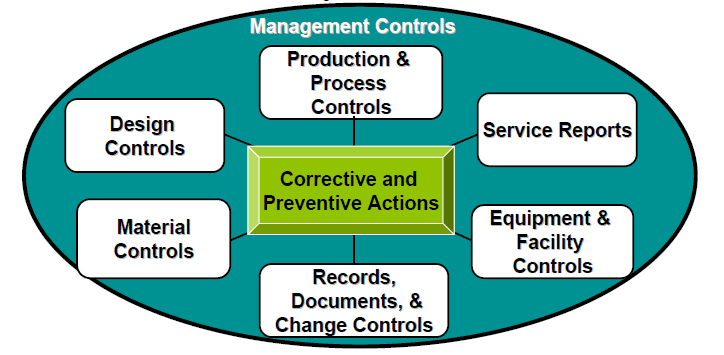
Corrective Action and Preventive Action (CAPA) is the critical component of QMS in the pharmaceutical industry. CAPA processes are designed to identify, investigate, and address the root causes of deviations, non-conformances, and other quality issues to ensure continuous improvement and prevent a recurrence. Let’s take a closer look at Corrective Action and Preventive Action in the pharmaceutical industry:
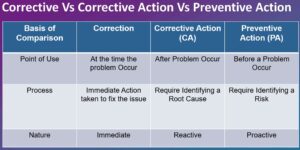
difference between corrective and preventive action
Corrective Action (CA):
Corrective Action is the process of identifying and addressing the root causes of identified non-conformances, deviations, or other quality issues in a timely and effective manner. It involves investigating the issue, determining the underlying causes, and taking appropriate actions to correct the problem and prevent its recurrence. CA focuses on addressing the immediate concern and ensuring that the issue is resolved promptly to prevent any further impact on product quality, patient safety, or regulatory compliance.
Preventive Action (PA):
Preventive Action is the process of identifying and addressing potential issues or risks before they occur to prevent their occurrence in the future. It involves proactive measures to identify and eliminate the root causes of potential deviations, non-conformances, or quality issues to avoid their recurrence. PA aims to identify and mitigate risks and implement preventive measures to ensure that quality issues are prevented from occurring in the first place, rather than just correcting them after they have occurred.
Key Steps in CAPA Process:
The CAPA process typically involves the following key steps:
Issue Identification: Identify and document any deviation, non-conformance, or quality issue that needs corrective or preventive action.
Investigation and Root Cause Analysis: Conduct a thorough investigation to determine the root causes of the issue using various tools and techniques, such as process mapping, fishbone diagrams, and 5 Whys analysis.
Corrective Action: Develop and implement appropriate corrective actions to address the identified root causes and resolve the immediate issue. This may involve process improvements, corrective measures, or retraining personnel.
Preventive Action: Identify and implement preventive measures to address potential risks or issues that may arise in the future. This may involve process enhancements, risk assessments, or the implementation of preventive controls.
Documentation and Verification: Document all CAPA activities, including the investigation, and corrective and preventive actions taken, and verify their effectiveness through appropriate monitoring and measurement.
Review and Closure: Review the effectiveness of the implemented CAPA and close the CAPA once the identified issue is resolved, and preventive measures are in place.

Benefits of CAPA in the Pharmaceutical Industry:
The implementation of a robust CAPA process in the pharmaceutical industry offers several benefits, including:
- Improved Product Quality: CAPA helps identify and address the root causes of quality issues, leading to improved product quality and compliance with regulatory requirements.
- Enhanced Patient Safety: CAPA helps prevent quality issues that could potentially impact patient safety, ensuring that pharmaceutical products are safe and effective for patient use.
- Continuous Improvement: CAPA promotes a culture of continuous improvement by identifying and addressing issues in a proactive manner, leading to enhanced processes and systems.
- Regulatory Compliance: CAPA helps ensure compliance with regulatory requirements, including Good Manufacturing Practices (GMP) and other quality standards.
- Risk Mitigation: CAPA helps identify and mitigate risks that may impact product quality, patient safety, or regulatory compliance, reducing the likelihood of recurrence.
- Cost Savings: CAPA helps identify and eliminate root causes of quality issues, leading to reduced rework, scrap, and product recalls, resulting in cost savings.
In conclusion, Corrective Action and Preventive Action (CAPA) are critical components of quality management systems in the pharmaceutical industry. Implementing a robust CAPA process helps identify and address the root causes of quality issues, ensures continuous improvement, enhances patient safety, and promotes regulatory compliance.
Why is CAPA important in the pharmaceutical industry?
Answer: CAPA is essential in the pharmaceutical industry because it helps ensure that any issues or deviations from established processes are addressed promptly and effectively. It is a proactive approach that focuses on identifying the root causes of problems and implementing corrective and preventive actions to prevent a recurrence. CAPA helps pharmaceutical companies maintain the quality, safety, and regulatory compliance of their products, which is crucial for patient safety and satisfaction. It also helps companies continually improve their processes, improving operational efficiency and reducing the risk of product recalls, regulatory actions, and financial losses.
What are the key steps in the CAPA process in pharmaceuticals?
Answer: The key steps in the CAPA process in pharmaceuticals are: the identification of issues, root cause analysis, corrective action, preventive action, verification, effectiveness review, and documentation and reporting.
What are the benefits of implementing an effective CAPA process in the pharmaceutical industry?
Answer: The benefits of implementing an effective CAPA process in the pharmaceutical industry include improved product quality, compliance with regulatory requirements, continual process improvement, and reduction of risks and costs associated with quality issues.
What are the key steps in the CAPA process in pharmaceuticals?
Answer: The CAPA process in the pharmaceutical industry typically involves the following key steps:
- Identification of an issue or deviation: This step involves detecting any deviations, non-conformities, or problems through various quality management tools such as inspections, audits, investigations, complaints, or trending data.
- Investigation and root cause analysis: Once an issue is identified, a thorough investigation is conducted to determine the root cause(s) of the problem. This may involve collecting and analyzing data, conducting experiments, or using other problem-solving techniques to identify the underlying causes of the issue.
- Corrective action: Based on the root cause analysis, appropriate corrective actions are developed and implemented to address the immediate issue. Corrective actions are designed to eliminate or mitigate the root cause(s) of the problem and prevent a recurrence.
- Preventive action: In addition to corrective actions, preventive actions are also implemented to address the potential recurrence of similar issues in the future. Preventive actions are proactive measures aimed at improving processes, systems, or procedures to prevent problems from occurring.
- Verification and effectiveness review: Once corrective and preventive actions are implemented, the effectiveness of these actions is verified through monitoring, testing, or other methods. A review is conducted to assess the overall effectiveness of the CAPA process and to ensure that the issue has been fully resolved.
- Documentation and reporting: Throughout the CAPA process, thorough documentation is maintained to capture all the steps taken, decisions made, and outcomes achieved. This documentation is used for reporting purposes to regulatory agencies, management, and other stakeholders as required.
What are types of CAPA?
Answer: There are two main types of CAPA (Corrective and Preventive Action) in the pharmaceutical industry:
- Corrective Action: This type of CAPA focuses on addressing existing issues or non-conformities that have been identified through investigations or audits. Corrective actions are aimed at resolving the immediate problem and eliminating the root cause(s) of the issue to prevent a recurrence. Corrective actions may include changes to processes, procedures, equipment, or other relevant factors to correct the identified problem and bring the situation back to compliance.
- Preventive Action: This type of CAPA focuses on proactive measures to prevent potential issues or non-conformities from occurring in the future. Preventive actions are aimed at identifying and mitigating potential risks or weaknesses in processes, systems, or procedures to prevent problems before they happen. Preventive actions may include process improvements, training, additional controls, or other preventive measures to reduce the likelihood of issues occurring in the future.
Both corrective and preventive actions are critical components of a robust CAPA process in the pharmaceutical industry, working together to address existing problems and prevent potential problems from occurring, thereby ensuring product quality, safety, and compliance with regulatory requirements.
What are the 4 stages of CAPA?
Answer: The four stages of CAPA (Corrective and Preventive Action) in the pharmaceutical industry are:
- Identification: In this stage, issues, non-conformities, or problems are identified through various means such as internal audits, customer complaints, deviations, out-of-specification results, or other quality-related events. Identification involves thoroughly understanding the issue, documenting it, and recording relevant information, including the nature of the problem, its severity, and its potential impact on product quality and safety.
- Investigation and Root Cause Analysis: Once an issue has been identified, the next stage involves investigating the root cause(s) of the problem. This stage often requires a systematic and thorough analysis of all relevant data, documentation, and records to determine the underlying reasons behind the issue. Root cause analysis techniques such as fishbone diagrams, 5 Whys, or fault tree analysis may be used to identify the true cause(s) of the problem and not just the symptoms.
- Corrective and Preventive Action: Based on the findings from the root cause analysis, the next stage involves developing and implementing corrective and preventive actions. Corrective actions are focused on addressing the immediate issue and eliminating the root cause(s) to prevent recurrence. Preventive actions, on the other hand, are proactive measures aimed at preventing similar issues from occurring in the future. Corrective and preventive actions may involve changes to processes, procedures, equipment, training, or other relevant factors, and should be well-documented, implemented, and monitored for effectiveness.
- Verification and Effectiveness Review: After implementing corrective and preventive actions, the next stage involves verifying the effectiveness of these actions. This may involve monitoring and measuring the results of the implemented actions to ensure that the issue has been resolved and that there are no unintended consequences. Additionally, a review of the effectiveness of the CAPA process itself may be conducted to identify any areas of improvement. Proper documentation and reporting of the verification and effectiveness review are essential to demonstrate compliance and continuous improvement.
The four stages of CAPA are cyclical and iterative, as the effectiveness of the implemented actions should be monitored over time, and any further issues or non-conformities should be addressed through the CAPA process, ensuring ongoing product quality, safety, and compliance with regulatory requirements.

3 thoughts on “CAPA in Pharma (corrective and preventive action) 2026”