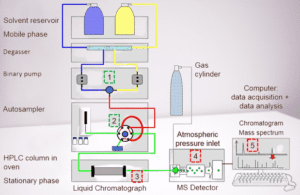
Chromatography plays a pivotal role in the pharmaceutical industry, difference between HPLC and GC techniques in pharmaceutical industries, where precise separation and analysis of compounds are crucial this way. Among the various chromatography techniques, High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) are the most widely used for their ability to separate complex mixtures into individual components. While both are used to achieve similar goals, their operational principles, equipment, and application areas vary significantly.
This article delves into the key differences between HPLC and GC, emphasizing their distinct roles in the pharmaceutical industry.
1. Mobile Phase
One of the primary differences between HPLC and GC lies in their mobile phases, which determine the conditions under which each method is applied.
- Gas Chromatography (GC): As its name suggests, GC utilizes a gaseous mobile phase, typically helium or nitrogen. The mobile phase carries the analyte through a column where separation occurs based on the interaction between the mobile phase and the stationary phase, which can be either liquid or solid.
- High-Performance Liquid Chromatography (HPLC): In contrast, HPLC uses a liquid mobile phase. This liquid can range from water and organic solvents to mixtures of both, depending on the nature of the analyte and the stationary phase. The liquid phase moves under high pressure, pushing the analyte through the stationary phase (usually solid) to facilitate separation.
The difference in mobile phases directly impacts the types of analytes that can be separated. GC is more suited for volatile or semi-volatile compounds, while HPLC can handle non-volatile, larger, and more complex molecules.
2. Nature of the Analyte
The nature of the analyte is another key distinction between GC and HPLC.
- GC: Gas Chromatography is ideal for analyzing volatile compounds, meaning substances that easily vaporize without decomposition. Examples of analytes suitable for GC include organic solvents, essential oils, and gases. Volatile compounds tend to be smaller in molecular weight and more thermally stable, as the separation process in GC occurs at elevated temperatures.
- HPLC: HPLC is designed to analyze non-volatile or less volatile compounds that may degrade at high temperatures. This includes larger molecules like proteins, peptides, and pharmaceuticals, which are often dissolved in the liquid mobile phase. As a result, HPLC is commonly used for a broader range of pharmaceutical applications, including drug testing and quality control of both liquid and solid formulations.
3. Column and Instrumentation
Though both techniques utilize chromatography columns, the size and structure of these columns vary considerably.
- GC Columns: Gas Chromatography columns are typically longer and narrower, ranging from 10 to 30 meters in length with an internal diameter of about 0.25 to 0.53 mm. This extended length allows for greater separation efficiency but requires higher temperatures for operation.
- HPLC Columns: HPLC columns are much shorter, generally 5 to 25 cm, with a wider diameter compared to GC columns. These shorter columns, paired with high-pressure pumps, allow for faster separations and the use of a liquid mobile phase. The stationary phase in HPLC columns is often packed with small particles to increase surface area and improve resolution.
4. Detection Methods
After separation, the next step is detecting and quantifying the individual compounds. GC and HPLC use different detection methods suited to their respective operational principles.
- GC Detectors: The two most common detectors in Gas Chromatography are the Flame Ionization Detector (FID) and the Thermal Conductivity Detector (TCD). FID is primarily used for detecting hydrocarbons and organic compounds due to its sensitivity to substances that ionize in a flame. TCD, on the other hand, is a universal detector that can detect any substance with a difference in thermal conductivity.
- HPLC Detectors: HPLC primarily uses Ultraviolet (UV) spectrometric detectors, where detection is based on the absorption of UV light by different compounds. As the analyte passes through the detector, the amount of light absorbed is measured, which helps quantify the concentration of the sample. UV detectors are highly sensitive to compounds that absorb light in the UV-visible range, making them ideal for many pharmaceutical compounds.
5. Cost and Complexity
Another significant difference between the two techniques is the cost and complexity of the equipment.
- GC: Gas Chromatography is generally less expensive than HPLC due to the simpler instrumentation and lower operational costs. GC systems do not require the high-pressure pumps or complex solvent systems that HPLC systems do. Thus, GC is often preferred in industries where cost is a significant factor, especially for volatile compounds.
- HPLC: HPLC systems, while more versatile, are also more expensive due to the use of high-pressure pumps and a wider range of solvent systems. The additional equipment required for HPLC also makes it more complex to operate, maintain, and troubleshoot, contributing to higher operational costs. However, its ability to handle a broader range of analytes justifies the investment for many pharmaceutical applications.
6. Applications in the Pharmaceutical Industry
- GC: In the pharmaceutical industry, GC is primarily used for analyzing residual solvents, volatile organic compounds (VOCs), and gases that may be present in drug formulations. It is a critical tool in ensuring that trace amounts of these solvents do not exceed safety limits.
- HPLC: HPLC, due to its versatility, is used for a much broader range of applications. It is employed for the analysis of active pharmaceutical ingredients (APIs), impurities, degradation products, and excipients in both research and quality control settings. HPLC is also instrumental in drug development, stability testing, and ensuring the purity and potency of pharmaceutical products.
Differences Between HPLC and GC in the Pharmaceutical Industry:
| Parameter | Gas Chromatography (GC) | High-Performance Liquid Chromatography (HPLC) |
|---|---|---|
| Mobile Phase | Gas (e.g., Helium, Nitrogen) | Liquid (e.g., Water, Organic Solvents) |
| Nature of Analyte | Volatile or Semi-Volatile Compounds | Non-Volatile, Larger, Complex Molecules |
| Column Size | Long (10-30 meters), Narrow | Short (5-25 cm), Wide |
| Operating Temperature | High Temperature | Room Temperature or Slightly Elevated |
| Detection Methods | Flame Ionization Detector (FID), Thermal Conductivity Detector (TCD) | Ultraviolet (UV) Spectrometry |
| Cost | Lower Initial and Operational Costs | Higher Costs Due to Pumps, Solvents, and Complexity |
| Analyte Types | Organic Solvents, Gases, Volatile Organic Compounds | APIs, Impurities, Excipients, Drug Products |
| Typical Applications | Residual Solvent Analysis, VOC Testing | Drug Development, Purity Testing, Stability Testing |
Conclusion:
Both HPLC and GC are indispensable techniques in the pharmaceutical industry, each serving unique roles based on the nature of the analyte and the required analysis. GC is the go-to method for volatile compounds, while HPLC offers broader versatility for more complex molecules. The decision to use one technique over the other depends on the specific requirements of the analysis, including cost, equipment, and the properties of the substances being analyzed.
Frequently Asked Questions:
What are the different types of detectors in HPLC?
HPLC detectors are broadly categorized into two types: specific detectors, such as Mass Spectrometry (MS), UV/VIS, and Fluorescence detectors, and bulk property detectors, like Refractive Index (RI), Electrical Conductivity, and Light Scattering detectors.
How many types of HPLC are there?
There are mainly two types of HPLC: Normal-phase HPLC and Reverse-phase HPLC, differentiated by the polarity of the stationary and mobile phases.
How many types of UV detectors are there?
There are three types of UV detectors used in HPLC: fixed wavelength detectors, variable wavelength detectors, and diode array detectors (DAD).
What are 3 uses of HPLC?
HPLC is widely used in:
- Pharmaceutical analysis for drug purity and formulation.
- Environmental testing for detecting pollutants in water and soil.
- Food and beverage quality control, ensuring consistency and safety.
What is the principle of HPLC?
HPLC works on the principle of partitioning between a stationary phase and a mobile phase, where compounds are separated based on their differing affinities for each phase.
What are the three types of detectors?
The three common types of detectors are:
- UV/VIS detectors
- Fluorescence detectors
- Mass spectrometric detectors
What is the principle of GC?
Gas Chromatography (GC) is based on the partitioning of analytes between a stationary phase and a mobile phase (carrier gas), separating compounds based on their boiling points and affinities for the stationary phase.
What is the full form of PDA detector?
PDA stands for Photodiode Array Detector, which can simultaneously detect absorbance over a wide range of wavelengths.
What is the DAD detector in HPLC?
A DAD (Diode Array Detector) is a type of UV/VIS detector that allows the detection of multiple wavelengths simultaneously, providing spectral information about analytes.
What is the RI detector in HPLC?
An RI (Refractive Index) detector measures the change in the refractive index of the mobile phase as analytes pass through, commonly used for compounds that do not absorb UV light.
What is the principle of HPLC UV detector?
The principle of a UV detector in HPLC involves measuring the absorption of ultraviolet light by compounds, where the amount of absorbed light correlates with the concentration of the analyte.
How many types of HPLC detectors are there?
There are several types of HPLC detectors, generally grouped into specific detectors (UV, MS, Fluorescence) and bulk property detectors (RI, Electrical Conductivity).
What is RF and RRF in HPLC?
RF (Response Factor) is a value that relates the detector response to the concentration of an analyte, while RRF (Relative Response Factor) is used to compare the detector response of different analytes relative to a reference compound.
What is a CAD detector in HPLC?
A Charged Aerosol Detector (CAD) is a mass-sensitive detector that measures non-volatile analytes by converting the eluent into charged aerosol particles.
What is the best detector in HPLC?
The “best” detector depends on the application, but MS detectors are considered the most sensitive and selective, especially for trace analysis.
What is RF detector in HPLC?
An RF detector refers to a Radiofrequency detector, used in specialized cases like inductively coupled plasma mass spectrometry (ICP-MS), but is not a common HPLC detector.
Which detector is used in HPTLC?
In High-Performance Thin-Layer Chromatography (HPTLC), densitometers and scanning UV detectors are commonly used to detect separated compounds on the plates.
How to calculate RRT?
Relative Retention Time (RRT) is calculated by dividing the retention time of an analyte by the retention time of a reference compound.
What is RRT impurity?
An RRT impurity refers to an impurity in a sample whose retention time is expressed as a ratio relative to the retention time of the main compound.
How to calculate impurity in HPLC?
Impurities in HPLC are calculated by comparing the area under the curve (AUC) of the impurity peak to the AUC of the main compound, often expressed as a percentage.
What is the principle of RI detector?
The RI detector in HPLC works on the principle of measuring the change in refractive index of the mobile phase as analytes elute, which alters the bending of light passing through the sample.
What are the two types of HPLC?
The two main types of HPLC are Normal-phase HPLC, where the stationary phase is polar and the mobile phase is non-polar, and Reverse-phase HPLC, where the stationary phase is non-polar and the mobile phase is polar.
Which lamp is used in HPLC?
Deuterium lamps are commonly used in HPLC for UV detection, providing a broad spectrum of UV light from 160 to 400 nm. Tungsten lamps are also used for visible light detection in the 400 to 800 nm range.