Dry heat sterilization is a very commonly used process in sterile pharma plants. The most affordable and well-liked sterilizing method for medical supplies and equipment is dry heat sterilization. Microorganisms are destroyed during steam sterilization when they are exposed to pressurized steam. Dry heat sterilization uses temperatures as high as 370ºC to inactivate bacteria, which must be at least 170ºC. The thermal elimination of difficult-to-eliminate thermally resistant pollutants, such as endotoxin or pyrogen, makes use of sterilization. dry heat sterilization methods are one of the sterilization types. 
The dry heat sterilizing procedure involves the conduction of heat. Heat is absorbed by the exterior of the device, which spreads throughout the entire construction until the desired temperature is reached. By oxidizing molecules, dry heat kills microorganisms.
Process of Dry heat sterilization (DHS)
Heat is transferred by conduction during the process of dry heat sterilizing. The device’s exterior surface absorbs heat, which then moves throughout the entire structure until the desired temperature is reached. Microorganisms are destroyed by dry heat by oxidizing molecules. The essential cell constituents are destroyed and the organism dies. The temperature is maintained for almost an hour to kill the most difficult of the resistant spores
Dry heat sterilization, which uses hot air to kill or incapacitate all life inside an industrial oven’s chamber, is one of the most useful and favored ways of sterilizing.
The oldest technique for sterilization was a dry heat. It offers a high degree of predictability and may be customized to fit a number of different sterilizing needs.
dry heat sterilization temperature :
Dry heat sterilization uses temperatures as high as 370ºC to inactivate bacteria, which must be at least 170ºC.
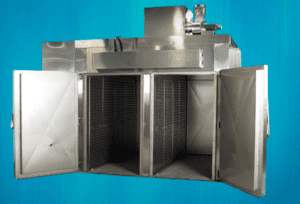
Working Principle of Dry heat sterilization:
principle of dry heat sterilization, depending on the temperature used, uses greater temperatures of up to 300°C and requires exposure times of up to 2 hours. The thing that needs to be sterilized is exposed to dry air, which conducts energy to it (forced air). Instead of fans, an oven might also employ heated coils (static air), although forced air is preferable since it delivers the heat load to the object more evenly. Mostly DHS use for the sterilization of ampoules and vials after that these ampoules transfer to the Ampoule Filling and Sealing Machine
The airflow in tunnels used for dry heat sterilization and depyrogenation should be planned to protect the performance and integrity of the grade. A sterilizing zone can be generated by maintaining the proper pressure differentials and airflow across the tunnel. It is important to assess the air pressure difference’s profile. The impact of the airflow change should be assessed to ensure that the heating profile is maintained. Every air entering the tunnel should be filtered with a minimum HEPA filter, and the efficiency of the filters should be periodically tested.
Proteins on all bacterial spores, fungi, viruses, prions, and essentially all forms of biological agents are being denatured by the heat applied to the object. All living things effectively perish from energy deprivation due to denaturation, whereas viruses are rendered permanently inactive due to severe damage to the RNA or DNA that encodes them. It is crucial to exclude any moisture from the air blown inside the oven chamber since this moisture can obstruct the denaturation of proteins.

Advantages of Dry Heat Sterilization (DHS):
- Dry heat ovens are usually inexpensive to purchase.
- Heating cycles and running costs are often affordable.
- The heat has the ability to sterilize materials thoroughly by penetrating them deeply. In this manner, even items contained in packaging can be sterilized.
- At high temperatures, metallic items with good heat resistance can be disinfected quickly.
- As dry heat has insufficient moisture, it won’t corrode metallic materials.
- Since no poisonous agents are used in the process, no hazardous materials will be dumped into the environment.
- Operates without the presence or assistance of a human. • The item may be removed and used nearly immediately because it cools down quickly, but someone has to set the oven and leave it to finish the cycle.
Disadvantages of Dry Heat Sterilization Compares to other Processes:
- Compared to the time needed for sterilization by steam, flame, chemical sterilization, or radiation, the dry heat can take much longer. Sensitive materials or thin sheets may deform as a result of heat.
- Plastics and rubber are inappropriate for dry heat due to the high temperatures’ potential for permanent damage.
- Even for materials that can withstand it, excessive heat exposure can cause some compounds’ chemical structures to change unintentionally.
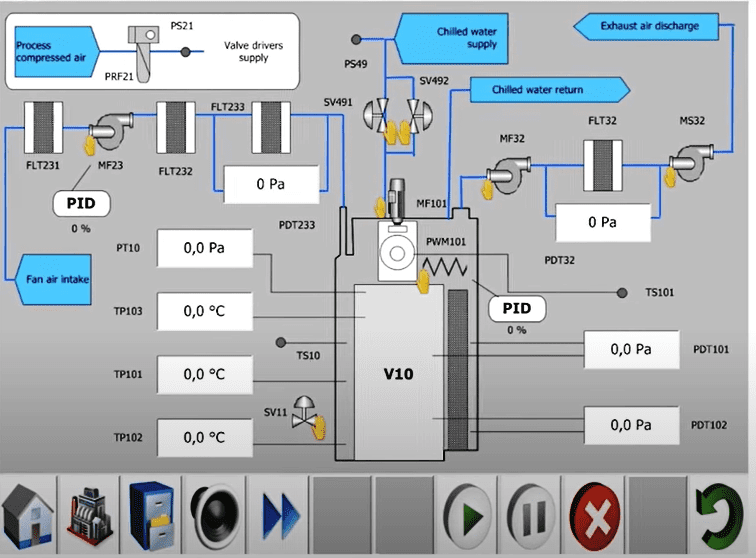
Application of Dry Heat Sterilization (DHS):
- Glass containers, ampoules, vials, and bottles
- Aluminum and stainless steel containers
- Talcum powder and sulphonamides are powders.
This approach is very common and employed because a large variety of materials can withstand dry heat well. Examples include all types of metals, inert powders that are unaffected by chemicals or moisture, anhydrous oils and fats, and glass. Additionally, and because dry heat can permeate objects so thoroughly, paper-wrapped products as well as medical instruments with complex geometries like syringes (metal and glass) or surgical tools can be sterilized successfully.
Despatch provides a large choice of sterilizing ovens with different chamber diameters and temperature ranges. Please get in touch with us if you need more details about any of our ovens, including oven design, sales, installation, and maintenance services.
Accessories Used in DHS and Its Function:
A full selection of carriages and transfer trolleys to make handling simpler.
Depending on the use, plates, trays, wire baskets, and other necessary accessories are provided.
- The chamber is made of SS316L. complete welding and rounded corners.
- The hinged doors feature a double-edge seal gasket for enhanced safety.
- A PLC is offered for use in operation, control, and data collection. HMI simplifies the interface between the operator and the machinery.
- Equipment with a single-door or a double-door pass-through arrangement is available.
- A bio seal flange can be fitted to seal the equipment into the building wall.
- The exhaust, recirculation, and fresh air modules all have HEPA filters installed. The Fresh Air HEPA also has a 0.5 micron “Micro B” filter installed as a pre-filter.
- Three air-handling modules
- Intake of fresh air
- Circulation
- Exhaust
Validation and documentation:
Complete documentation and certification traceable to national and international standards:
- MOC / COA certificate of contact and noncontact PARTS.
- MOC food grade and nontoxic certificates of contact and noncontact gaskets
- Electrical diagram
- Sets of maintenance and operational manual
- Functional specification and design specification (FS/DS)
- Calibration certificates of gauges and instruments with traceability certificate
- Operational manual of automated or PLC system / MMI
- Operational & maintenance manuals for the coater.
- Maintenance Manual
- PLC input/output list
- PLC and control panel wiring diagram
- List of alarm and their rectification procedure
- List of functional authorization
- FAT / SAT document
- GA (General alignment) drawing
- Schematic diagram of the machine showing overall dimension (as built drawing)
- System architecture diagram configuration and calibration certificate
- Alarm list (diagnostic and system created)
- FAT and installation qualification protocol
- Flameproof certificate of motor and accessories
- Certificate of HEPA filter and test certificate and certificate of another filter
- P&ID (Piping and instrumentation diagram)
- PLC backup
- Complete set of DQ, and IQ Documents.
- All documents are as per URS
Manufactures Company in India:
- Pharmalab India Ltd.
- Despatch thermal Process technology
- Raphanal System
- Systec Laboratory
- Eqnitron Medica Pvt. Ltd.
- Bionics Scientific Technologies (P) Ltd.
Frequently Asked Questions for Dry Heat Sterilization (DHS):
How does dry sterilization work?
what is dry heat sterilization?
Answer: Dry heat sterilization employs extremely high temperatures—at least 170°C—to inactivate germs. Due to the high temperatures encountered during sterilization, dry heat kills microorganisms through oxidation (cell bursting). Depending on the temperature used, it uses greater temperatures of up to 300°C and requires exposure times of up to 2 hours.
What is the primary dry heat sterilization technique?
Answer: Heat is transferred by conduction during the process of dry heat sterilizing. The device’s exterior surface absorbs heat, which then moves throughout the entire structure until the desired temperature is reached. Microorganisms are destroyed by dry heat by oxidizing molecules. This whole process is dry heat sterilization.
What uses dry heat sterilization?
Answer: For non-wettable goods, such as glassware, oils, powders, metal equipment, and items wrapped in paper, dry heat sterilization is employed.
What is the name of the dry heat sterilization machine?
Answer: For pharmaceutical and related industries, laboratories, research institutes, the food industry, etc., dry heat sterilizers and depyrogenation units are perfect. They are made for sterilization that is both safe and effective, including depyrogenation of rubber stoppers, medical powder, tablets, ampoules, and vials.
Example of DHS dry heat sterilization?
Answer: Typical Uses for Dry Heat Sterilization
Examples include all types of metals, inert powders that are unaffected by chemicals or moisture, anhydrous oils and fats, and glass.
Which materials are used for dry heat sterilization?
Answer:
| Material | Maximum Temperature |
| Nylon(Polyamide, heat-stabilized grades) | Up to 266⁰F |
| Metal | Up to 190°Ca |
| Muslin | Up to 160°C |
| High-density polyethylene | Up to 120°C |
| Aluminum | Up to 190⁰C |
| Silicon | Up to 210⁰ C |
What are the 4 methods of sterilization?
Answer: High-pressure steam (autoclave), dry heat (oven), chemical sterilants (glutaraldehydes or formaldehyde solutions), physical agents, or high heat can all be used to achieve this (radiation). Sterilization is a process, not an event, hence all steps must be followed precisely for sterilization to take place.
What do you mean by Sterilization?
Answer: The fundamental purpose of sterilization is to eliminate all types of germs and their spores. It is done to keep the environment sanitary. Typically, combinations of filtration, heat, irradiation, high pressure, etc. are used to accomplish this.
Describe the fundamentals of sterilization.
Answer: An article, object, or medium may be sterilized using physical or chemical methods to remove microorganisms.
What is the moist heat sterilization principle?
Answer: The rationale behind wet heat sterilization is that a high temperature causes the germs’ proteins to congeal, thus killing them.
What category does sterilisation fall under?
Answer: Sterilization techniques come in two flavors: chemical and physical.

1 thought on “Dry Heat Sterilization Easy Working & Principle 2023”