A lyophilizer, also known as a freeze dryer, is a device used to remove moisture from a substance by freeze-drying or sublimation. It is commonly used in various industries such as pharmaceuticals, food processing, and research laboratories. The lyophilization process involves freezing the substance and then applying a vacuum to remove the ice through sublimation, converting it directly from a solid to a vapor without passing through a liquid state. This preserves the substance’s structure, integrity, and bioactivity, making it suitable for long-term storage and transportation. Lyophilizers are essential for producing dried products with extended shelf life and maintaining the stability of sensitive materials.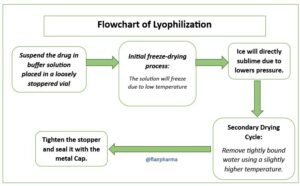
Working Principle of Lyophilizer
The working principle of a lyophilizer machine, or freeze dryer, involves several stages:
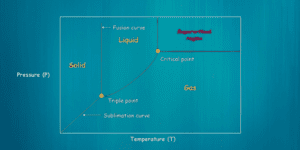
- Freezing: The substance to be dried is placed in the lyophilizer’s chamber, and the temperature is lowered to below its freezing point. This freezing step solidifies the water content within the substance.
- Vacuum: Once the substance is frozen, a vacuum is applied to the chamber. The low pressure reduces the surrounding air’s moisture content and creates a pressure gradient, allowing for sublimation to occur.
- Sublimation: As the vacuum is applied, the frozen water within the substance transitions from a solid state directly to a vapor state without passing through a liquid phase. This process is known as sublimation. The water vapor is removed from the chamber, leaving behind a freeze-dried product.
- Desorption: After most of the water has sublimed, desorption is used to remove any residual moisture. This is achieved by gradually raising the temperature of the chamber, causing the remaining water molecules to desorb and be removed.
- Final drying: To ensure the complete removal of moisture, the temperature and pressure conditions are adjusted accordingly to achieve the desired dryness level for the substance. This step helps in extending the shelf life and stability of the dried product.
Throughout the lyophilization process, it is important to carefully control the temperature, pressure, and time parameters to ensure efficient and effective drying while preserving the substance’s integrity. Monitoring and adjusting these variables allows for customization based on the specific requirements of the material being processed.
Key Components of a Lyophilizer
Lyophilizer instrument
A lyophilizer, or freeze dryer, typically consists of several key components. Here are the essential components found in a typical lyophilizer:
- Chamber: The chamber is the main enclosure where the substance to be dried is placed. It is typically made of stainless steel and is designed to withstand low temperatures and vacuum conditions. The chamber provides a controlled environment for the freeze-drying process.
- Condenser: The condenser is a critical component that cools and collects the water vapor sublimated from the frozen substance. It consists of a refrigeration system that maintains a low temperature, typically below freezing point, allowing the water vapor to condense into solid ice. The condenser helps to remove moisture from the chamber, preventing it from re-entering the substance during the drying process.
- Vacuum System: The vacuum system creates and maintains a low-pressure environment inside the lyophilizer’s chamber. It typically includes a vacuum pump that removes air and other gases, reducing the pressure within the chamber. The vacuum system facilitates the sublimation of water by lowering the boiling point and promoting efficient drying.
- Heating System: The heating system is responsible for controlling the temperature within the lyophilizer. It may include heating elements or coils strategically placed within the chamber to raise the temperature during the desorption phase, facilitating the removal of residual moisture from the dried product.
- Control System: The control system consists of sensors, controllers, and software that monitor and regulate various parameters such as temperature, pressure, and time. It allows operators to set and adjust the desired drying parameters based on the specific requirements of the material being processed. The control system ensures that the lyophilization process is carried out accurately and efficiently.
- Shelves or Trays: Shelves or trays are used to hold the containers or trays containing the substance to be dried. These shelves are typically made of stainless steel and are evenly spaced within the chamber. They provide a surface for the substance to be frozen and dried while allowing proper air circulation.
- Refrigeration System: The refrigeration system cools the condenser and maintains its temperature below freezing point. It typically consists of a compressor, condenser coils, and refrigerant. The refrigeration system is crucial for removing heat from the condenser, facilitating the condensation of water vapor.
These are the primary components found in a lyophilizr. However, the specific design and configuration may vary depending on the size, capacity, and application of the lyophilizr.
Lyophilization Process
Critical Parameter of a Lyophilizer
| Parameter | Description | Typical Range |
|---|---|---|
| Chamber Temperature | The temperature of the shelves holding the product. | -50°C to +60°C |
| Shelf Temperature | The temperature of the condenser surface for water vapor condensation. | -50°C to +70°C |
| Condenser Temperature | The maximum amount of material that can be loaded into the chamber. | -50°C to -80°C |
| Vacuum Level | Pressure level maintained inside the chamber. | Below 10 mTorr |
| Drying Time | Duration of the drying process. | Hours to several days |
| Product Load Capacity | The maximum amount of material that can be loaded into the chamber. | Varies based on size |
| Ice Condensing Capacity | The rate at which the condenser temperature is reduced for freezing. | Varies based on size |
| Heating Rate | The capacity of the condenser to collect and freeze water vapor. | Adjustable |
| Cooling Rate | Rate at which the condenser temperature is reduced for freezing. | Adjustable |
| Control and Monitoring | The rate at which the chamber temperature is increased during desorption | Included |
Lyophilizer’s 3 Primary Stages
A lyophilizer, or freeze dryer, typically undergoes three primary stages during the freeze-drying process. These stages are:
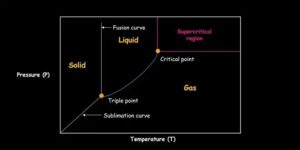
- Freezing: In this stage, the substance to be dried is placed in the lyophilizer’s chamber, and the temperature is lowered to below the substance’s freezing point. Freezing solidifies the water content within the substance, forming ice crystals. This stage is crucial for preserving the structure and integrity of the material.
- Primary Drying (Sublimation): Once the substance is frozen, the lyophilizer creates a vacuum by removing the surrounding air and reducing the chamber’s pressure. The vacuum allows for sublimation to occur. Sublimation is the process where the frozen water within the substance transitions directly from a solid state to a vapor state without passing through the liquid phase. The ice crystals sublimate, converting into water vapor and leaving behind a freeze-dried matrix. This stage removes the majority of the moisture from the substance.
- Secondary Drying (Desorption): After the primary drying stage, some residual moisture may still remain within the freeze-dried material. In the secondary drying stage, the temperature of the chamber is increased, causing the remaining water molecules to desorb or evaporate from the material. Desorption ensures that the material is thoroughly dried and free of moisture, enhancing its stability and extending its shelf life.
It is important to note that each stage requires precise control of temperature, pressure, and time to achieve optimal freeze-drying results. Monitoring and adjusting these parameters ensure the successful removal of moisture while preserving the quality and integrity of the material being processed.
Applications of Lyophilizer
| Industry | Application |
| Pharmaceuticals | – Preservation of vaccines, proteins, and enzymes. – Production of injectable drugs in powder form. – Creation of stable pharmaceutical formulations. – Long-term storage of labile drugs and biologicals. |
| Food Processing | – Production of freeze-dried coffee, fruits, and herbs. – Preservation of sensitive food ingredients. – Creation of lightweight and compact food products. – Retention of nutritional properties in food. |
| Research | – Sample preparation for electron microscopy |
| Laboratories | – Preservation of laboratory samples and cultures – Drying sensitive and fragile materials. – Storage of biological samples for long-term use. – Preparation of reagents and standards |
| Biotechnology | – Freeze-drying of enzymes, probiotics, and cultures. – Preservation of diagnostic kits and reagents. – Production of stable biopharmaceuticals. – Long-term storage of genetic material |
| Cosmetic Industry | – Drying of botanical extracts and ingredients. – Creation of powdered cosmetic formulations. – Preservation of sensitive cosmetic products. – Extended shelf life of cosmetic ingredients – Creation of powdered cosmetic formulations. |
| Chemical Industry | – Drying of chemical compounds and intermediates. – Production of powdered chemicals and reagents. – Creation of stable chemical formulations. – Long-term storage of sensitive chemicals. |
Lyophilizer manufacturers
There are several lyophilizer manufacturers worldwide, including some based in India. Here are a few prominent lyophilizer manufacturers globally and in India:
Worldwide:
- SP Scientific: SP Scientific is a well-known manufacturer of laboratory equipment, including lyophilizers. They offer a range of lyophilization systems for various applications.
- Millrock Technology, Inc.: Millrock Technology specializes in the design and manufacturing of advanced freeze-drying equipment, including lyophilizers, for pharmaceutical, biotechnology, and research applications.
- GEA Group: GEA Group is a global supplier of process engineering solutions. They offer lyophilizrs for applications in the pharmaceutical, biotechnology, and food industries.
- Labconco Corporation: Labconco Corporation is a manufacturer of laboratory equipment, including freeze-drying systems. They provide a range of lyophilizrs for research and industrial applications.
In India:
- Lyophilization Systems India Pvt. Ltd. (LSIPL): LSIPL is an Indian company specializing in the design, manufacturing, and servicing of lyophilizers and freeze-drying equipment for the pharmaceutical, biotechnology, and food industries.
- Freeze Tech Equipments Pvt. Ltd.: Freeze Tech Equipments is an Indian manufacturer and exporter of freeze-drying equipment, including lyophilizrs. They offer customized solutions for various industries.
- Optima Pharma Pvt. Ltd.: Optima Pharma is an Indian company that manufactures pharmaceutical machinery, including lyophilizrs. They provide lyophilization solutions for the pharmaceutical and biotechnology sectors.
Lyophilizer validation protocol
The validation of a lyophilizer, or freeze dryer, involves a series of steps to ensure that the equipment performs reliably and consistently in accordance with the desired specifications. While specific protocols may vary depending on regulatory requirements and industry standards, here are general steps that are typically included in a lyophilizer validation protocol:
- Installation Qualification (IQ):
- Verify that the lyophilizer is installed correctly as per the manufacturer’s instructions.
- Check and record equipment specifications, including power requirements, dimensions, and operating conditions.
- Validate that all necessary utilities (power, water, etc.) are available and properly connected.
- Operational Qualification (OQ):
- Perform a comprehensive functional test of the lyophilizer’s components, including vacuum system, heating system, refrigeration system, and control system.
- Ensure that the lyophilizer operates within specified temperature and pressure ranges.
- Validate the accuracy and reliability of sensors, gauges, and controls.
- Verify that alarms and safety features are functional and appropriately calibrated.
- Performance Qualification (PQ):
- Conduct performance testing using representative products or materials.
- Verify that the lyophilizer achieves the desired freezing and drying parameters, such as shelf temperature, condenser temperature, vacuum level, and drying time.
- Monitor and record critical process parameters during multiple lyophilization cycles.
- Assess the uniformity and consistency of freeze-dried products.
- Cleaning and Sterilization Validation:
- Develop and implement a cleaning and sterilization validation protocol to ensure the lyophilizer can be effectively cleaned and sanitized.
- Verify the effectiveness of cleaning procedures, including residue removal and reduction of microbial contamination.
- Documentation and Reporting:
- Document all validation activities, including procedures, results, and observations.
- Generate a final validation report summarizing the validation process, findings, and conclusions.
- Ensure that all validation documentation complies with regulatory requirements and internal quality standards.
It’s important to note that the validation process may be subject to specific regulations and guidelines based on the industry and intended use of the lyophilizer. Consulting applicable regulatory authorities and industry standards can provide further guidance on specific validation requirements.
Frequently Asked Questions
What is the purpose of a lyophilizer machine?
Answer: A lyophilizer machine is used to remove moisture from a substance through freeze-drying, preserving its structure and integrity.
How does a lyophilizer achieve freezing of the substance?
Answer: The lyophilizer lowers the temperature of the substance below its freezing point using a cooling system.
What is the function of the condenser in a lyophilizer?
Answer: The condenser collects and freezes the water vapor sublimated from the substance, removing it from the chamber.
What is the significance of applying a vacuum in a lyophilizer?
Answer: The vacuum lowers the pressure inside the chamber, facilitating the sublimation of water and efficient drying.
What is the purpose of the heating system in a lyophilizer?
Answer: The heating system is used during the desorption stage to raise the temperature, aiding in the removal of residual moisture.
How is the drying time determined in a lyophilizer?
Answer: The drying time is determined based on the specific material being processed, its moisture content, and desired dryness level.
Can a lyophilizer handle different sample sizes?
Answer: Yes, lyophilizers can be designed to accommodate a range of sample sizes, from small vials to large-scale production batches.
What is the typical range of vacuum level in a lyophilizer?
Answer: The vacuum level in a lyophilizer is typically maintained below 10 mTorr.
How is the shelf temperature controlled in a lyophilizer?
Answer: The shelf temperature is controlled using heating elements or cooling coils in direct contact with the shelves.
Can the lyophilizer’s parameters be adjusted during the drying process?
Answer: Yes, the lyophilizer’s parameters such as temperature, pressure, and time can be adjusted and optimized for specific materials.
How is the cooling rate of the condenser controlled?
Answer: The cooling rate of the condenser is controlled by adjusting the refrigeration system’s settings, including the compressor and condenser coils.
What safety features are typically included in a lyophilizer machine?
Answer: Safety features may include alarms for temperature deviations, pressure relief valves, and automated shutdown systems in case of emergencies.
Can lyophilizers handle heat-sensitive materials?
Answer: Yes, lyophilizers are suitable for drying heat-sensitive materials as the freeze-drying process is carried out at low temperatures.
Is it possible to monitor and control a lyophilizer remotely?
Answer: Yes, some lyophilizer models offer remote monitoring and control capabilities through integrated software or connectivity options.
What maintenance is required for a lyophilizer machine?
Answer: Regular maintenance includes cleaning, checking, and replacing seals, inspecting refrigeration components, and calibrating sensors and controls.
You may also read about Sterility Test.

I needed to thank you for this great read!!
I definitely enjoyed every little bit of it. I have got you saved as a favorite to look at new things
you post
Your contribution helps us continue producing high quality content material that educates, conjures up, and drives optimistic change.
thank you very much