NVPC i.e. Non-viable particle monitoring plays a pivotal role in numerous industries, ensuring quality control and maintaining clean environments. In this article, we delve into the depths of this crucial practice, exploring its modern trends, methodologies, and advancements. Brace yourself for an immersive journey through the intricate world of non-viable particle monitoring.
Importance of the Non-Viable Particle Monitoring In The Pharma
Non-viable particle monitoring ( NVPC ) plays a pivotal role in the pharmaceutical industry, serving as a linchpin for regulatory compliance, product quality assurance, process optimization, and patient safety. By implementing robust monitoring systems and embracing technological advancements, pharmaceutical plants can proactively detect and mitigate particle contamination, ensuring the production of high-quality medications. As the industry continues to evolve, the importance of non-viable particle monitoring remains unwavering, underscoring its critical role in upholding the integrity of pharmaceutical products and safeguarding the well-being of patients worldwide.
Regulatory Compliance and Quality Assurance:
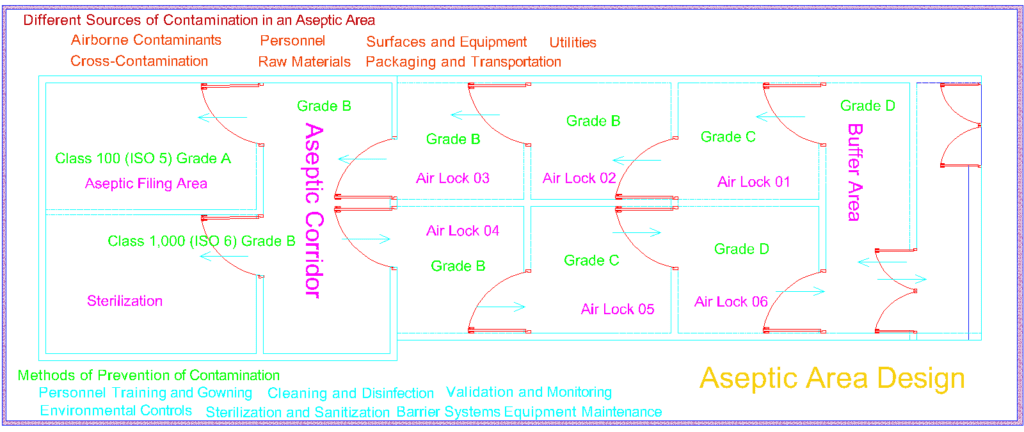
Pharmaceutical plants operate in a highly regulated environment, governed by stringent guidelines and standards. Non-viable particle monitoring ( NVPC ) serves as a cornerstone in meeting these regulatory requirements, specifically in areas such as cleanrooms, controlled environments, and aseptic processing areas. By implementing robust monitoring systems, pharmaceutical plants can demonstrate compliance with regulatory bodies and validate the efficacy of their quality control measures.
Maintaining Product Quality:
The presence of contaminants, such as non-viable particles, can jeopardize the quality and efficacy of pharmaceutical products. These particles may originate from various sources, including human activity, equipment, or ambient surroundings. (NVPC) Non-viable particle monitoring plays a critical role in detecting and quantifying these particles, enabling swift corrective actions to mitigate potential risks. By ensuring the cleanliness of manufacturing environments and minimizing particle contamination, pharmaceutical plants can uphold the highest standards of product quality and integrity.
Process Optimization and Efficiency:
( NVPC) Non-viable particle monitoring not only serves as a quality control measure but also contributes to process optimization and efficiency. By continuously monitoring particle levels and trends, pharmaceutical plants can identify areas for improvement and implement preventive measures to minimize particle generation. This proactive approach enhances manufacturing efficiency, reduces product defects, and streamlines production processes. Furthermore, by leveraging data analytics and real-time monitoring, plants can gain valuable insights into particle behavior, aiding in the optimization of cleaning protocols and the identification of potential sources of contamination.
Patient Safety:
Above all, the ultimate goal of non-viable particle monitoring in pharmaceutical plants is to safeguard patient safety. Contaminated products can pose significant risks to patients, leading to adverse reactions, compromised efficacy, or even life-threatening consequences. By rigorously monitoring and controlling non-viable particle levels, pharmaceutical plants can ensure the delivery of safe and effective medications to patients, instilling confidence in the healthcare system and upholding the trust placed in the pharmaceutical industry.
Regulatory Guidance and Limits for Monitoring (NVPC) Non-Viable Particle Counts: USFDA and EUGMP Standards
Regulatory authorities such as the US Food and Drug Administration (USFDA) and the European Union Good Manufacturing Practice (EUGMP) provide guidelines and limits for non-viable particle monitoring in the pharmaceutical industry. This article presents a table outlining the regulatory guidance and limits for monitoring non-viable particle counts as per USFDA and EUGMP standards.
EUGMP: Annex 1 of the EUGMP provides guidelines for the classification of cleanrooms and controlled environments in pharmaceutical manufacturing. The following table outlines the limits for particle counts in different classification grades of the NVPC.
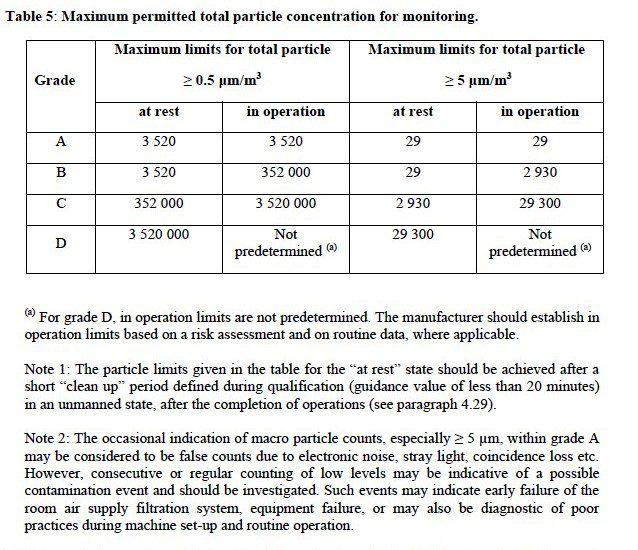
USFDA: The USFDA provides guidance for the industry on cleanroom classifications based on the International Organization for Standardization (ISO) standards fo the nvpc. The following table outlines the limits for particle counts in different ISO classes and grades.
| ISO Class / Grade | Particle Size (≥0.5 μm) | Particle Size (≥5 μm) |
|---|---|---|
| ISO 5 / Grade A | < 3,520 | < 20 |
| ISO 7 / Grade B | 352,000 | < 3,520 |
| ISO 8 / Grade C | 3,520,000 | < 35,200 |
| ISO 9 / Grade D | N/A | 352,000 |
Components of a typical Non-Viable Particle Counter ( NVPC ) :
| Component | Description |
|---|---|
| Aerosol Sampler | A device that draws in air samples from the environment for analysis, capturing particles for subsequent measurement. |
| Flow Control System | Regulates the airflow rate through the particle counter, ensuring a consistent and controlled sample volume. |
| Optical Sensor | Utilizes optical principles, such as light scattering or light obscuration, to detect and count particles in the air sample. |
| Particle Detection System | A module that analyzes the optical signals from the sensor and converts them into particle counts and size distributions. |
| Data Acquisition System | Collects and records the particle count and size data obtained from the detection system for further analysis and reporting. |
| Display and User Interface | Notifies users of abnormal particle levels or system malfunction through visual or audible alarms, ensuring timely response and intervention. |
| Sample Collection Unit | Provides a visual representation of particle count data, and system status, and allows users to interact with the particle counter. |
| Calibration System | Enables calibration and verification of the particle counter to ensure accurate and reliable measurements. |
| Data Storage and Export | Allows for the storage of particle count data and the export of data files in various formats for documentation and analysis purposes. |
| Alarm and Alert System | Notifies users of abnormal particle levels or system malfunctions through visual or audible alarms, ensuring timely response and intervention. |
| Power Supply | Provides electrical power to the particle counter for its operation. |
Frequently Asked Questions:
What is the NVPC in pharma?
Answer: NVPC stands for Non-Viable Particle Count in the pharmaceutical industry. It refers to the measurement and monitoring of the concentration of non-living or inanimate particles present in the air within a controlled environment.
What is non viable particle monitoring?
Answer: ( NVPC ) Non-viable particle monitoring involves the continuous surveillance and analysis of airborne particles that do not possess the ability to grow, reproduce, or sustain life. These particles can include dust, fibers, lint, and other contaminants that may be present in the pharmaceutical manufacturing environment.
What is viable and non viable air monitoring?
Answer: Viable and ( NVPC ) non-viable air monitoring refers to the distinction between the measurement and monitoring of living particles (viable) and non-living particles (non-viable) in the air. Viable air monitoring focuses on the detection and enumeration of microorganisms such as bacteria and fungi, which have the potential to impact product quality, while non-viable air monitoring aims to identify and control non-living particles that may pose risks to product integrity and patient safety.
Why only 0.5 and 5 micron particle count required in pharma?
Answer: In the pharmaceutical industry, particle counts are typically performed at specific particle size ranges, such as 0.5 microns and 5 microns. These size ranges are chosen based on their relevance to pharmaceutical manufacturing processes and their association with potential product contamination. Contaminants within these size ranges are considered critical due to their ability to potentially impact product quality and safety.
What is the limit of non-viable particle count?
Answer: The limit for non-viable particle count in pharmaceutical environments is determined based on regulatory guidelines, industry standards, and specific manufacturing requirements. These limits vary depending on factors such as the cleanroom classification, the type of pharmaceutical product being manufactured, and the intended use of the controlled environment.
Why do we use 0.3 micron filters?
Answer: 0.3 micron filters are commonly used in pharmaceutical manufacturing processes for air filtration and particle control. This particular filter size is selected due to its efficiency in capturing particles of various sizes, including both larger and smaller particles. The 0.3 micron filter size is often regarded as a challenge size, as it is near the most penetrating particle size for filters and is therefore considered a conservative measure for ensuring effective particle removal. Using 0.3 micron filters helps maintain the cleanliness and integrity of the manufacturing environment by reducing the presence of particles that may impact product quality.
Why is non-viable air monitoring important in pharmaceutical manufacturing?
Answer: (NVPC) Non-viable air monitoring is essential in pharmaceutical manufacturing to ensure the control and prevention of airborne contamination. By monitoring non-viable particles, such as dust, fibers, and other contaminants, pharmaceutical companies can maintain the cleanliness and integrity of their manufacturing environments, minimizing the risk of product contamination and ensuring product quality and safety.
How can non-viable air monitoring data be used for process improvement?
Answer: Non-viable air monitoring data can be analyzed and utilized for process improvement initiatives. By identifying trends or deviations in particle counts, manufacturers can pinpoint potential sources of contamination and implement corrective actions. This data-driven approach helps optimize manufacturing processes, enhance operational efficiency, and strengthen quality control practices to minimize the risk of product contamination and improve overall production outcomes.
How often should non-viable air monitoring be performed?
Answer: The frequency of ( NVPC ) non-viable air monitoring depends on several factors, including the cleanroom classification, the type of pharmaceutical product being manufactured, and regulatory requirements. Generally, routine monitoring is conducted at regular intervals, such as daily, weekly, or monthly, to ensure ongoing control of airborne particles. Additionally, non-viable air monitoring should be performed during critical manufacturing processes or after events that may impact air quality, such as maintenance or equipment changes.
You may also read about:

1 thought on “NVPC Monitoring in Pharma 2023”