Pharma Project Work Flow by Flair Pharma:
The pharmaceutical industry in India is the world’s largest supplier of generic drugs, and it is well-known for its low-cost vaccines and generic medications. The Indian pharmaceutical industry is currently ranked third in pharmaceutical production by volume, having evolved over time into a thriving, growing industry.
Also, the pharmaceutical industry in India is an important part of the country’s foreign trade and offers lucrative opportunities for investors and business personnel. So that’s why flair pharma provides complete solutions & knowledge base for awareness.
Conceptualization and Designing of New Pharma Facility:
- The pharma project’s salient features, structure, and major deliverables are conceptualized and planned during the early phase of project discussion.
- Designs are developed to meet all the stakeholder’s requirements as well as the local and international regulatory requirements.
Execution with Innovative and optimum Solutions:
- Self-motivated and professional team having the knowledge to identify any probable risks which may directly or indirectly impact the project.
- After identification certation of a detailed plan with the assessment of potential risk to ensure desired output from the project as intended to fulfill all Regulatory and Customer Requirements.
Steps to ward the best implementation of project setup:
- Project Monitoring & Control.
- Pharma Project Completion.
- Pharma Project Execution Flow.
- Pharma Project Architectural, Civil, and structural Designing.
- Pharma Project Mechanical, Utilities, and Piping work:
- Pharma Project Plant equipment & Utility equipment:
1.0 Project Monitoring & Control:
Establishment of the project baselines which includes the project charter, the scope of work, the project schedule, and the tentative project budget. This helps the seamless execution of all planned tasks.
2.0 Pharma Project Completion:
Before the launch of the project, detailed planning will do which includes project completion on time within budget, by ensuring sufficient resources are in place for each critical aspect of the project and work distribution to specified technical team members.
3.0 Pharma Project Execution Flow:
- Pharma Project Process Design:
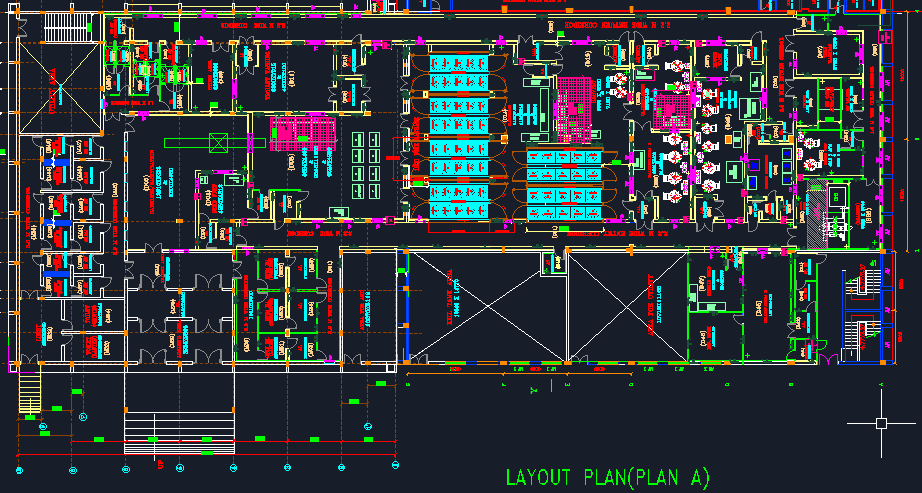
- Site plan, Facility/Plant Conceptualization Layout Designing
- Preparation of PIDs for process and utilities.
- Preparation of a detailed layout for the process plant and utilities.
- Preparation of design bases.
4.0 Pharma Project Architectural, Civil, and structural Designing:
- Preparation of Detailed Master plot plan.
- Complete set of civil and structural (RCC, structural steel, CRP) design and construction drawings for buildings, foundations of equipment, cable racks, pipe racks, doors, windows, finishes, plumbing, etc. for new setup/ additional jobs.
- Full infrastructure (Indoor/Outdoor) development plan.
- Complete outer road & drain development design.
5.0 Pharma Project Mechanical, Utilities, and Piping work:
- Detailed and described design for piping and instrumentation for the process equipment and utilities.
- Preparation of design basis documents.
- Preparation of piping layout.
- Preparation of detailed specifications for equipment, piping, insulation, fittings, etc.
- Preparation of drawings for statutory approval.
6.0 Pharma Project Plant equipment & Utility equipment:
- Preparation of URS documents.
- Preparation of FAT documents.
- Preparation of SAT documents.
- Preparation of DQ documents.
- Preparation of IQ documents.
- Preparation of OQ documents.
- Preparation of PQ documents.
Pharma Project Electrical work:
- Preparation of single line diagram (SLD).
- Preparation of design basis documents.
- Preparation of HT/LT substation layout and drawings.
- Electrical layout for lighting, power, earthing, etc., and route plan for power cable, control cable, etc.
- Preparation of technical specifications for various equipment like transformers, power control centers, motor control centers, distribution boards, lighting fixtures/fittings, cables, D.G. sets, etc.
Pharma Project Automation /Instrumentation:
- Optimized Process Control and instrumentation.
- QC and Micro Lab designing.
- Preparation of design basis documents.
- Instruments list with detailed specifications.
- Review and approve vendors’ detailed engineering drawings and specifications.
- Preparation of cable schedule, and tray/trench details.
Pharma Project HVAC System (Airconditioning & Ventilation):
- Preparation of Room data sheet for the system as per process requirement.
- The basic design for the system is as per process/clean room requirements.
- Preparation of tender document.
- Preparation of and analysis of (TBA)Technical Bid Analysis.
- Preparation and analysis of (CBA) Commercial Bid Analysis.
Pharma Project Fire Safety:
- Preparation of basic data for fire & safety system requirements and providing the inputs to the vendors for Design, supply, and installation of the same as per the process requirement.
- Technical Evaluation of the offers for a selection of the vendor.
Pharma Project Effluent Treatment Plant (ETP):
- Preparation of basic design data as per the process.
- The overall package of designing the complete setup of (ETP) Effluent Treatment Plant that complies with all the statutory requirements.
- Analysis, reviewal, and approval of vendor’s and OEM’s detailed engineering drawings and technical specifications.
Pharma Project Post-Project Completion Services:
- Complete integrated pharma project designing & commissioning implementation. The primary emphasis is to cater through effective Systems & Services to the Specific requirement of the facility as per applicable GMP/GLP/GSP standards.
Layouts for Pharma Facilities Expansion/Modification to Meet current cGMP/GMP, GLP, and regulatory requirements
- Ensure Process Equipment Size and outline basic Specification.
- Define Environment (Clean Room) Classification and Pressure Differential.
- Water Treatment System Establishment.
- Layouting of quality control lab and microbiology lab.
- ETP/STP System Establishment.
- Budgetary Project Cost (Tentative).
- Project Timeline (Tentative).
- Facility (Plant Machinery Qualification/Validation).
- GMP Audit and GAP Analysis.
- GMP/GLP/QMS Documentation Support.
