Validation in pharma industrial plants is a process of creating official documentation proving that a procedure, process, or activity used in testing and ultimately manufacturing maintains the appropriate degree of compliance at all times is known as validation in the pharmaceutical industry. In the pharmaceutical sector, it is crucial to guarantee that the process will consistently deliver the desired results in addition to final product testing and compliance. The desired outcomes are determined in terms of the process’s intended outcomes. As a result, systems and equipment qualification is a step in the validation process. Food, drug, and pharmaceutical regulatory organizations like the US FDA and their good manufacturing practices guidelines all require validation. Due to the necessity for numerous different procedures, operations, and activities.
As we are working in pharmaceutical companies so, have worked diligently to manufacture life-saving drugs.
They have made people live longer, more fulfilling lives.
Pharma Equipments:
- Equipments are the bone marrow of any pharmaceutical plant. The pharmaceutical industry has the most precise requirements and manufacturing guidelines in terms of quality. As a result, it is critical that pharmaceutical manufacturing equipment complies with good manufacturing practices (GMP).
- Production equipment should only be used within its qualified operating range.
MOTO: Zero Breakdown, Zero defective product, Safe environment & Easy Operation
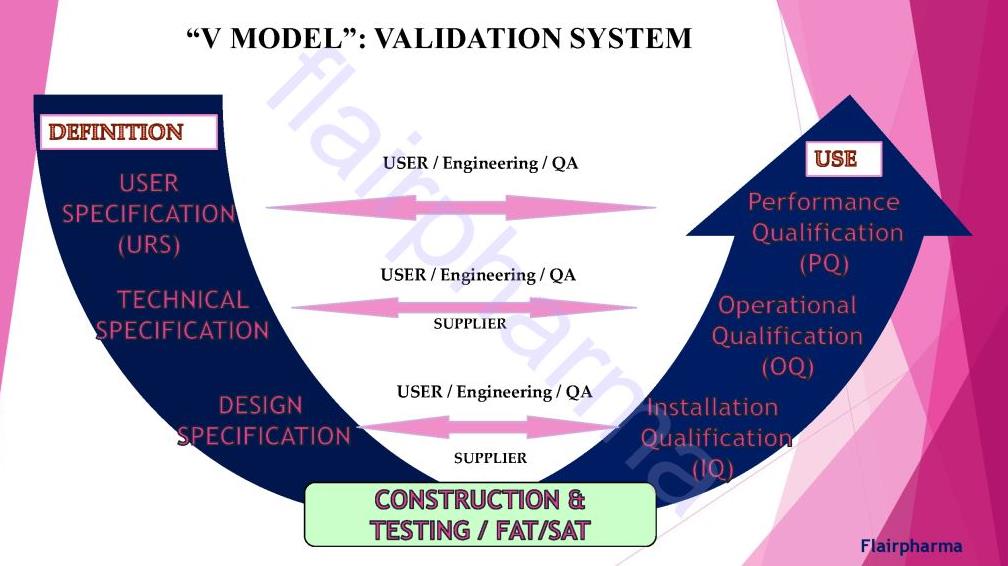
Types of validation in pharma:
The topic of validation is divided into several subsections since it is necessary to validate a wide range of procedures, processes, and activities. The followings are the categorizations:
- Area Validation.
- Plant Equipment validation.
- Validation of analytical methods.
- CSV Validation of computer systems.
- CV Cleaning validation.
- Validation of the HVAC system.
- Validation of utility systems.
- Water System validation.
- Microbiological Method Validation.
- Validation of analytical methods.
- Validation of computerized systems.
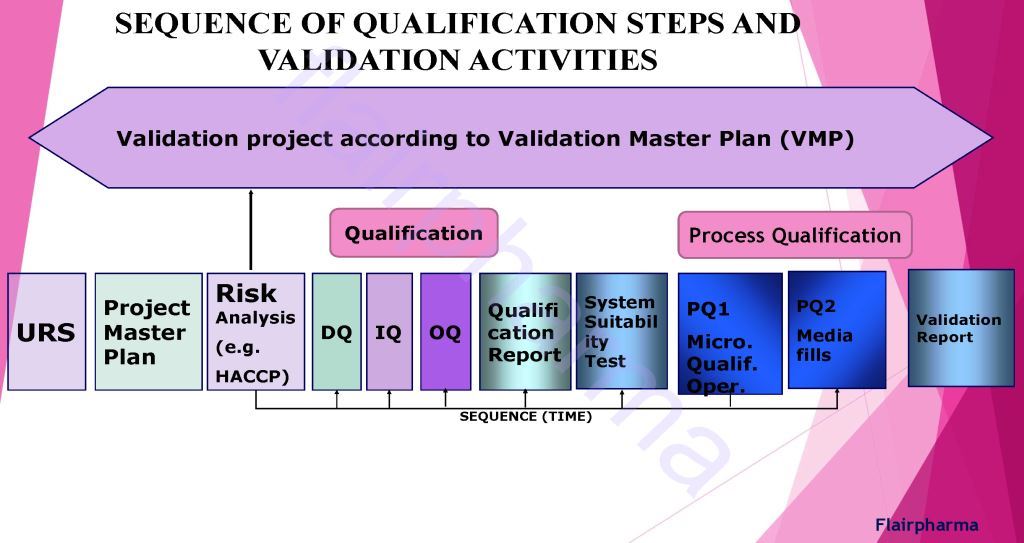
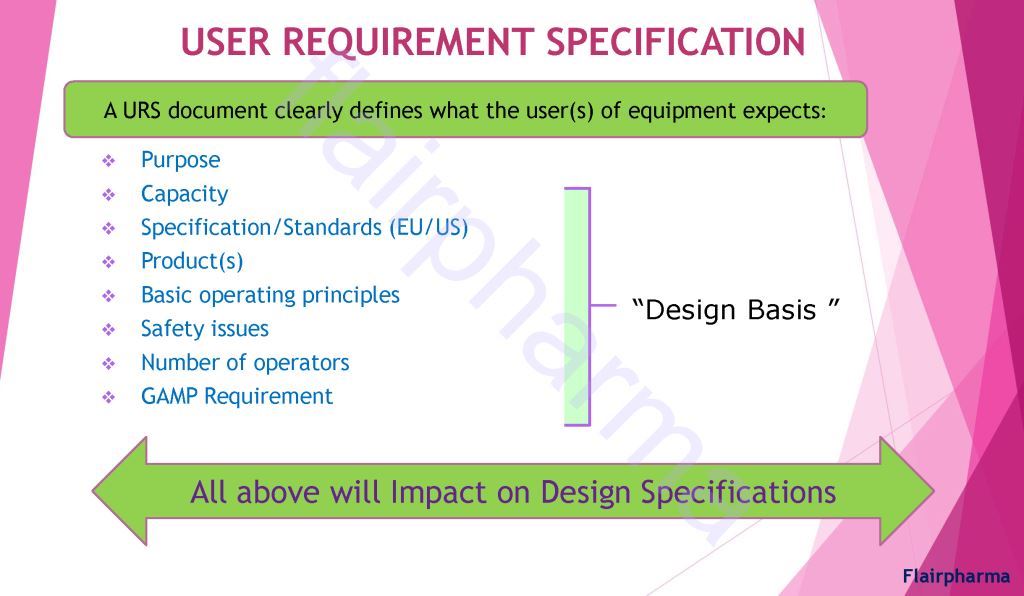
Validation in pharma has similar divisions made in the activity of qualifying systems and equipment, which include the following: - URS User Requirement Specification
- FAT Factory Acceptance Test.
- SAT Site Acceptance Test
- DQ Design Qualification
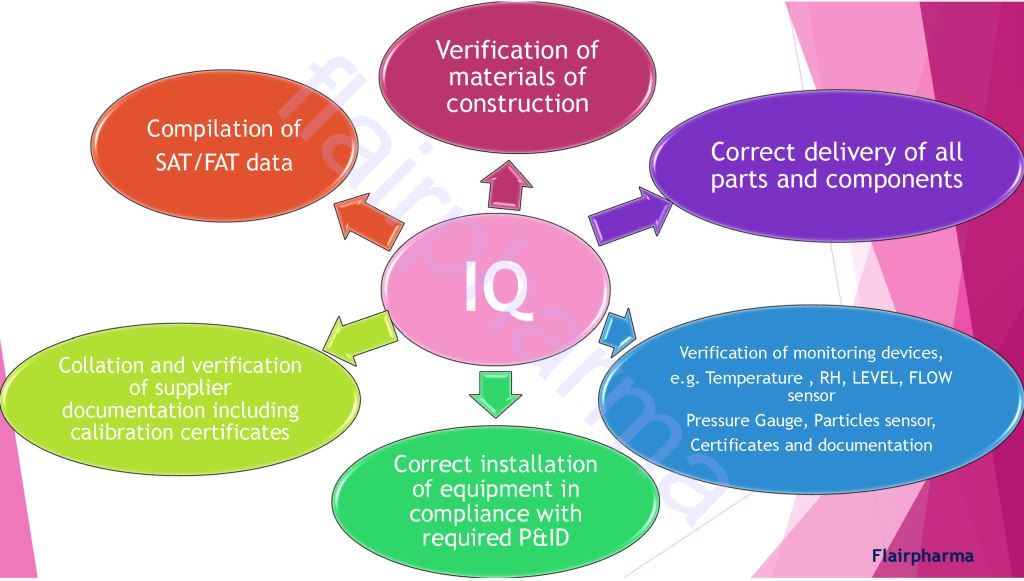
- IQ Installation Qualification
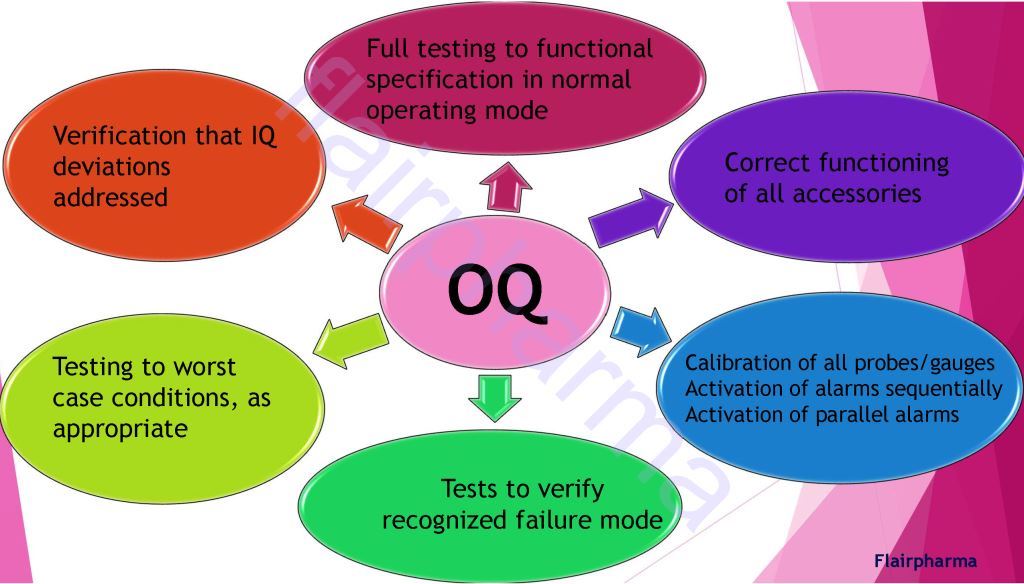
- OQ Operational Qualification
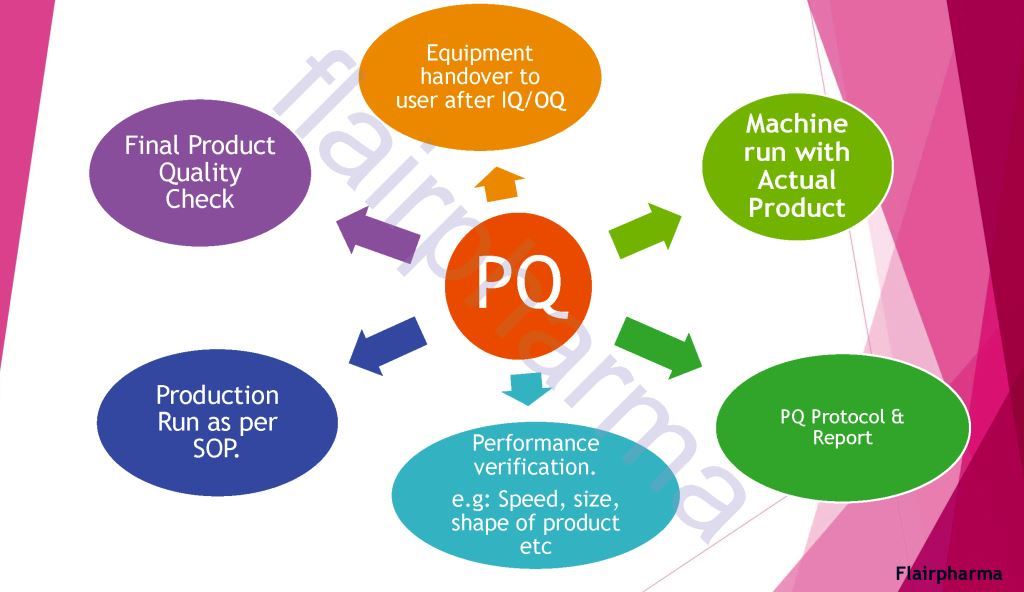
- PQ Performance Qualification
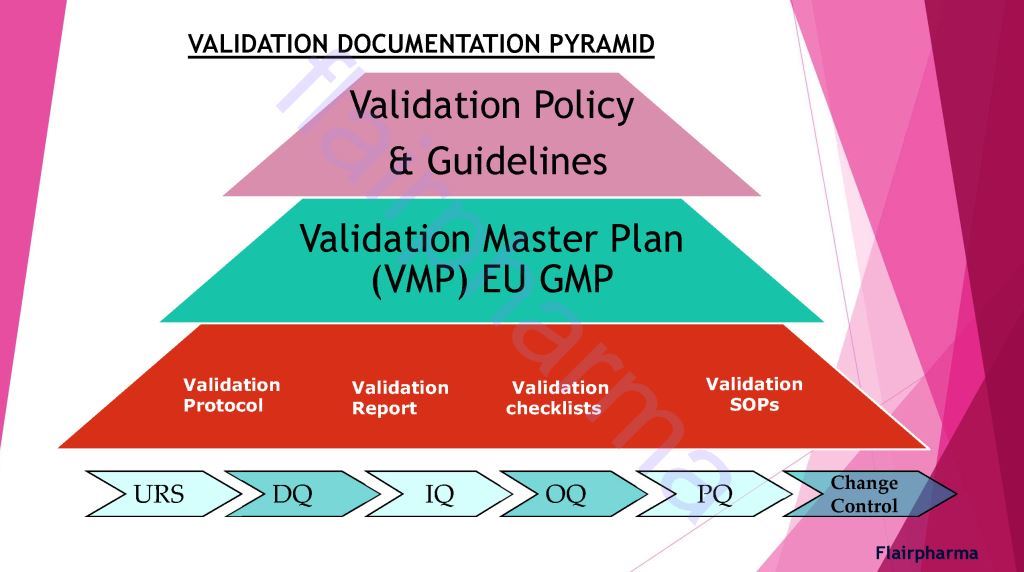
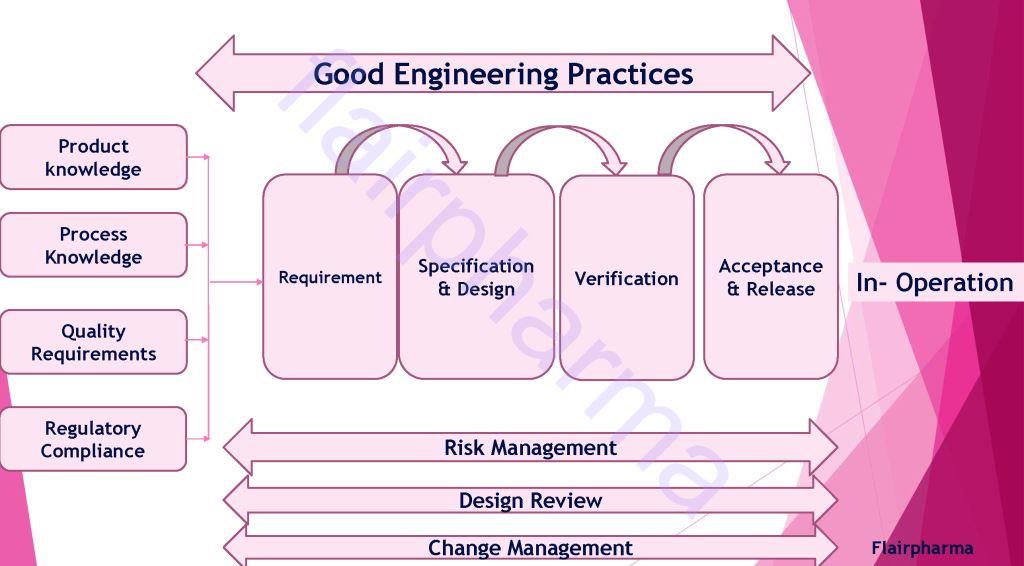
The sequence of Validation In pharma:
Difference between validation and Qualification:
Validation is the checks to make sure the chosen method will produce accurate results that are sufficient for the intended use. The validation involves a variety of parameters. Some of them are listed below:
- Accuracy.
- Specification.
- Reproducibility.
- Linearity.
- Range.
- Detection Limit.
- Repeatability.




Application of Equipment Validation in pharma:
The pharmaceutical industry places a high value on equipment validation.
- Equipment validation lowers costs by lowering rejections, reworks, and downtime.
- Reduce the possibility of regulatory non-compliance.
- Good client satisfaction rate.
- Methods and calibrations for analytical testing are carried out.
- It also lessens testing of the finished product and work-in-progress.
- Increase employee awareness as well.
- Make equipment maintenance simpler.
- Provide more dependable and quick startup for new equipment.
- Assist in creating the facility’s validation master plan.
- In the event of an inspection, the validation documentation may be utilized as a presentation. (As evidence and proof).

You have a way of making each of your readers feel seen and heard That’s a special quality that not all bloggers possess Thank you for creating a safe space for us