
Container Closure Integrity Test (CCI) testing is an essential part of the aseptic filling process in pharmaceutical plants. CCIT ensures that the container and its closure maintain the intended barrier properties throughout the shelf life of the drug product, protecting it from contamination and degradation.
Main Purpose of CCIT:
The main purpose of CCI testing is to identify any potential leaks or defects in the container or closure system that may compromise the integrity of the drug product. CCIT testing is typically performed using non-destructive methods that do not compromise the sterility of the drug product, such as:
- Visual inspection: The container and closure system are visually inspected for any visible defects or damage.
- Leak testing: The container and closure system are subjected to a pressure differential or vacuum, and any leaks are detected by measuring the resulting pressure changes.
- High voltage leak detection: This method uses high voltage to create a current across the container and closure system. Any leaks or defects in the system will disrupt the current and trigger an alarm.
- Mass extraction: This method involves extracting the headspace gas from the container and analyzing it for any trace amounts of the drug product. Any detected drug product indicates a leak or defect in the container or closure system.
The CCI testing method chosen will depend on the specific requirements of the drug product and the container and closure system being used. It is essential to establish appropriate CCI testing procedures and specifications during the drug product development process and to regularly monitor and review these procedures to ensure ongoing compliance with regulatory requirements and best practices in the pharmaceutical industry.

Key features of Container Closure Integrity Test (CCI) testing:
| Key Features of CCI Testing | Description |
| Importance | There are several CCIT testing methods, including visual inspection, leak testing, high-voltage leak detection, mass extraction, and bubble testing. The appropriate method(s) will depend on the specific drug product and container/closure system being used. |
| Testing Methods | There are several CCIT testing methods, including visual inspection, leak testing, high-voltage leak detection, mass extraction, and bubble testing. The appropriate method(s) will depend on the specific drug product and container/closure system being used. |
| Risk-Based Approach | A risk-based approach to CCIT testing is important to ensure that the appropriate level of testing is conducted based on the potential risks associated with the drug product and container/closure system. |
| Validation | CCIT testing methods must be validated to ensure they are appropriate and effective for the specific drug product and container/closure system being used. |
| Regulatory Requirements | CCIT testing is a regulatory requirement for pharmaceutical manufacturers and must be conducted in compliance with applicable regulations, such as USP <1207> and FDA guidance. |
| Sampling Plan | A representative sampling plan must be established to ensure that the testing accurately reflects the overall quality of the container/closure system. |
| Sensitivity | The testing method must be sensitive enough to detect potential leaks or defects that could compromise the integrity of the drug product. |
| Accuracy | The testing method must be accurate and reliable to provide consistent and repeatable results. |
| Personnel Training | Personnel conducting CCIT testing must be properly trained and qualified to ensure that the testing is conducted correctly and consistently. |
| Combination of Methods | A combination of non-destructive and destructive testing methods may be necessary for comprehensive CCIT testing. |
| Container/Closure System Compatibility | The testing method must be compatible with the container/closure system being used to ensure accurate and effective testing. |
| Ongoing Monitoring | CCIT testing should be an ongoing process that is regularly monitored and reviewed to ensure ongoing compliance with regulatory requirements and best practices in the pharmaceutical industry. |
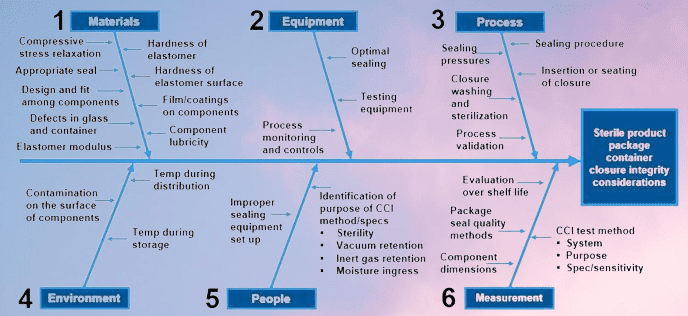
Key Area in the CCI Testing:
| Area | Description | Importance |
| Materials | The container/closure system, drug product, and testing materials used in CCI testing | Ensuring that materials used in CCI testing are compatible and effective in detecting potential leaks or defects |
| Equipment | The equipment used in CCI testing, such as testing machines or visual inspection equipment | Ensuring that the equipment used is appropriate and maintained to perform accurate and reliable testing |
| Process | The testing procedures, methods, and validation of CCI testing | Ensuring that the testing procedures and methods are appropriate, effective, and validated for the specific drug product and container/closure system |
| Environment | The environmental conditions in which CCI testing is conducted, such as temperature, humidity, and air quality | Ensuring that the environmental conditions are controlled and monitored to prevent any potential impact on CCI testing |
| People | The personnel responsible for conducting CCI testing, including their training and expertise | Ensuring that personnel are adequately trained and have the necessary expertise to conduct accurate and reliable testing |
| Measurement | The methods and criteria used to measure and evaluate the results of CCI testing | Ensuring that the methods used to measure and evaluate CCI testing results are appropriate, accurate, and consistent with regulatory requirements |
Importance of Container Closure Integrity Test in pharma:
- CCI testing ensures that the container and closure system maintain the intended barrier properties throughout the shelf life of the drug product, protecting it from contamination and degradation.
- CCI testing helps to identify any potential leaks or defects in the container or closure system that may compromise the integrity of the drug product.
- CCI testing is an essential part of regulatory compliance for pharmaceutical manufacturers, as it is a requirement of various regulatory agencies such as the FDA and EMA.
- CCI testing helps to ensure patient safety by reducing the risk of contaminated or ineffective drug products reaching the market.
- CCI testing can help to reduce the risk of product recalls, which can be costly and damaging to a pharmaceutical company’s reputation.
- CCI testing is critical for maintaining the sterility of the drug product during the aseptic filling process.
- CCI testing is an ongoing process that should be regularly monitored and reviewed to ensure ongoing compliance with regulatory requirements and best practices in the pharmaceutical industry.
key features and advantages of Container Closure Integrity Test (CCI) in the aseptic filling of pharma plants:
| Key Features of CCI Testing | Advantages |
| Ensures container and closure system integrity. | Reduces the risk of contamination or degradation of the drug product. |
| Detects potential leaks or defects. | Allows for customization based on specific drug products and container/closure systems. |
| Non-destructive testing methods available. | Minimizes risk of compromising the sterility of drug product. |
| Allows for customization based on specific drug products and container/closure systems. | Allows for customization based on specific drug products and container/closure system. |
| Various testing methods are available. | Provides accurate and reliable data for process control and regulatory compliance. |
| Helps to reduce the risk of product recalls | Ensures compliance with FDA and EMA regulations. |
| Non-destructive testing methods are available. | Saves costs and protects company reputation. |
| An ongoing process that should be regularly monitored and reviewed. | Allows for customization based on specific drug products and container/closure systems. |
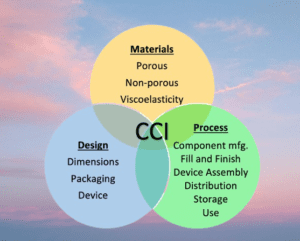
Container Closure Integrity Test by A Risk-Based Approach
Container Closure Integrity (CCI) A Risk-Based Approach refers to the practice of conducting CCI testing in a manner that is proportionate to the potential risks associated with a specific drug product and container/closure system. This approach involves assessing the risks associated with a drug product and its container/closure system and tailoring the CCIT testing accordingly to ensure that it is appropriate and effective in detecting potential leaks or defects that could compromise the integrity of the drug product.
The risk-based approach to CCIT testing recognizes that not all drug products and container/closure systems are created equal and that some may present greater risks to patient safety and product quality than others. As a result, the testing must be conducted in a manner that is consistent with the potential risks associated with the product and container/closure system to ensure that the appropriate level of testing is conducted. This approach helps to ensure that CCIT testing is conducted efficiently and effectively while also maintaining the safety and quality of the drug product.
Technical Points of Container Closure Integrity Test:
| Technical Points of CCI Testing | Description |
| Validation | CCI testing methods must be validated to ensure they are appropriate and effective for the specific drug product and container/closure system being used. |
| Sampling Plan | A representative sampling plan must be established to ensure that the testing accurately reflects the overall quality of the container/closure system. |
| Sensitivity | The testing method must be sensitive enough to detect potential leaks or defects that could compromise the integrity of the drug product. |
| Accuracy | The testing method must be accurate and reliable to provide consistent and repeatable results. |
| Training | Personnel conducting CCI testing must be properly trained and qualified to ensure that the testing is conducted correctly and consistently. |
| Regulatory Requirements | CCI testing is a regulatory requirement for pharmaceutical manufacturers and must be conducted in compliance with applicable regulations, such as USP <1207> and FDA guidance. |
| Ongoing Monitoring | CCI testing should be an ongoing process that is regularly monitored and reviewed to ensure ongoing compliance with regulatory requirements and best practices in the pharmaceutical industry. |
| Combination of Methods | A combination of non-destructive and destructive testing methods may be necessary for comprehensive CCI testing. |
| Container/Closure System Compatibility | The testing method must be compatible with the container/closure system being used to ensure accurate and effective testing. |
Frequently Asked Questions Container Closure Integrity Test:
What is Container Closure Integrity (CCI) testing?
Answer: CCI testing refers to the process of testing the integrity of a container/closure system to ensure that it can effectively protect the drug product from contamination and maintain its quality and stability.
What are some common CCI testing methods?
Answer: Some common CCI testing methods include visual inspection, leak testing, high voltage leak detection, mass extraction, and bubble test.
Why is CCI testing important in the pharmaceutical industry?
Answer: CCI testing is critical to ensuring the quality of drug products by maintaining the integrity of the container/closure system, and protecting the drug from contamination and degradation.
What are some factors that can impact the accuracy and reliability of CCI testing?
Answer: Factors that can impact the accuracy and reliability of CCI testing include the sensitivity of the testing method, the training of personnel conducting the testing, and the compatibility of the testing method with the container/closure system being used.
What is a risk-based approach to CCI testing?
Answer: A risk-based approach to CCI testing involves tailoring the testing to the potential risks associated with the drug product and container/closure system, to ensure that the appropriate level of testing is conducted.
How is CCI testing validated?
Answer: CCI testing methods are validated to ensure that they are appropriate and effective for the specific drug product and container/closure system being used. And ensure the Sterility testing.
What is the role of regulatory agencies in CCI testing?
Answer: Regulatory agencies, such as the FDA, require pharmaceutical manufacturers to conduct CCI testing in compliance with applicable regulations, such as USP <1207> and FDA guidance.
Can CCI testing be conducted on all types of container/closure systems?
Answer: The appropriate CCI testing method(s) will depend on the specific drug product and container/closure system being used, and the testing method must be compatible with the container/closure system being used to ensure accurate and effective testing.
What is the importance of a representative sampling plan in CCI testing?
Answer: A representative sampling plan is critical to ensuring that the testing accurately reflects the overall quality of the container/closure system.
Why is ongoing monitoring of CCI testing important?
Answer: Ongoing monitoring of CCI testing is important to ensure ongoing compliance with regulatory requirements and best practices in the pharmaceutical industry. It also helps to identify any potential issues or areas for improvement in the testing process.
