Boiling point apparatus provide crucial insights into the physical and structural properties of substances. Whether in academic research or industrial applications, accurately determining the boiling point of a liquid is fundamental. This is where the boiling point apparatus becomes indispensable. By enabling precise measurement of boiling points, this apparatus ensures that scientists and engineers can gather reliable data for various substances.
What is Boiling Point?
The boiling point of a liquid is defined as the temperature at which its vapor pressure equals the atmospheric pressure surrounding it, causing the liquid to transform into vapor. This temperature is crucial for understanding the behavior of liquids under different environmental pressures. For instance, at sea level, water boils at 100°C due to the atmospheric pressure being standard. However, in a vacuum or at higher altitudes, where the atmospheric pressure is lower, water boils at a significantly lower temperature.
Principle of Boiling Point Determination
The principle behind determining the boiling point involves heating a liquid until its vapor pressure matches the surrounding atmospheric pressure. This equilibrium between the liquid and gas phases marks the boiling point. The apparatus used for this purpose is designed to provide controlled heating and precise measurement, ensuring accurate and reproducible results.
Components of a Boiling Point Apparatus
A typical boiling point apparatus consists of several key components, each playing a vital role in the determination process:
- Heating Source: This is typically a Bunsen burner, an electric heater, or a hot plate that provides the necessary heat to bring the liquid to its boiling point.
- Boiling Flask: The liquid whose boiling point is to be determined is placed in this flask. It is usually made of heat-resistant glass to withstand high temperatures.
- Thermometer or Temperature Sensor: This is used to measure the temperature at which the liquid boils. Modern boiling point apparatuses often use digital sensors for enhanced accuracy.
- Condenser: This component cools the vapor back into liquid form, allowing for continuous boiling without loss of liquid. It ensures that the vapor does not escape and condenses back into the boiling flask of the boiling point apparatus.
- Manometer: In some advanced setups, a manometer is used to measure the pressure within the apparatus, which is especially useful for experiments conducted under non-standard atmospheric pressures.
- Control Panel: Modern apparatuses come with a control panel that allows the user to set and monitor various parameters, such as heating rate and temperature of the boiling point apparatus.

Procedure for Determining Boiling Point in Boiling Point Apparatus
- Preparation: The liquid sample is placed in the boiling flask. If necessary, boiling stones or chips are added to prevent superheating and ensure smooth boiling.
- Heating: The heating source is activated, gradually increasing the temperature of the liquid.
- Observation: The temperature is closely monitored using the thermometer or digital sensor. The point at which a steady stream of bubbles rises from the liquid to the surface, indicating that the vapor pressure is equal to the atmospheric pressure, is noted as the boiling point.
- Cooling: The condenser cools the vapor back into liquid form, which then returns to the boiling flask. This allows for continuous boiling without significant loss of liquid.
- Recording: The boiling point is recorded, and the experiment can be repeated to verify the accuracy and reproducibility of the measurement.
Importance of Boiling Point Apparatus
The boiling point apparatus is a fundamental tool in both educational and professional laboratories. Its precise measurements are critical for various applications:
- Identification of Substances: The boiling point is a characteristic property of pure substances and can be used to identify unknown compounds.
- Quality Control: In industrial settings, ensuring that a substance meets its specified boiling point is vital for quality assurance.
- Research and Development: Accurate boiling point data is essential for the synthesis of new compounds and the study of their properties.
Factors Affecting Boiling Point :
- Atmospheric Pressure: The boiling point varies with changes in atmospheric pressure. Higher altitudes with lower pressure result in lower boiling points.
- Purity of the Substance: Impurities in a liquid can raise or lower its boiling point, affecting the accuracy of the measurement.
- Intermolecular Forces: Substances with strong intermolecular forces, such as hydrogen bonds, have higher boiling points compared to those with weaker forces.
In conclusion, the boiling point apparatus is an essential instrument in scientific research and industrial applications. Its ability to provide precise and reliable measurements of boiling points underpins many experimental procedures and quality control processes. Understanding and utilizing this apparatus effectively allows scientists and engineers to gather crucial data, leading to advancements in various fields of study and industry.
Technical Specifications of the Boiling Point Apparatus
| Specification | Details |
| Boiling Flask | Heat-resistant glass, typically 50-250 mL capacity |
| Heating Source | Bunsen burner, electric heater, or hot plate |
| Temperature Range | Up to 300°C (varies based on the heating source and substance) |
| Thermometer/Temperature Sensor | Mercury or alcohol thermometer, digital temperature sensor, range: -10°C to 300°C |
| Condenser | Glass condenser, typically Liebig or reflux type |
| Manometer (optional) | Measures pressure, typically in mmHg or kPa, range: 0-760 mmHg |
| Control Panel | Digital or analog control panel for setting and monitoring temperature and pressure |
| Insulation | Insulated casing to minimize heat loss and ensure stable temperature |
| Boiling Stones/Chips | Inert, non-reactive materials to prevent bumping during boiling |
| Material of Construction | Stainless steel or high-quality plastic for durability and chemical resistance |
| Power Supply (if electric) | 220-240V, 50/60Hz for electric heaters, adjustable power output |
| Safety Features | Overheat protection, thermal cut-off, and safety shield for the heating source |
| Dimensions | Variable, typically compact design to fit standard laboratory benches (e.g., 30 x 30 x 50 cm) |
| Weight | Approx. 5-10 kg depending on the configuration and additional features |
| Accessories Included | Boiling stones, additional thermometers, spare parts for the heating element |
| Warranty | Typically 1-2 years depending on the manufacturer |
| Compliance | Meets relevant standards and certifications (e.g., ISO, CE) for laboratory equipment |
Calculations:
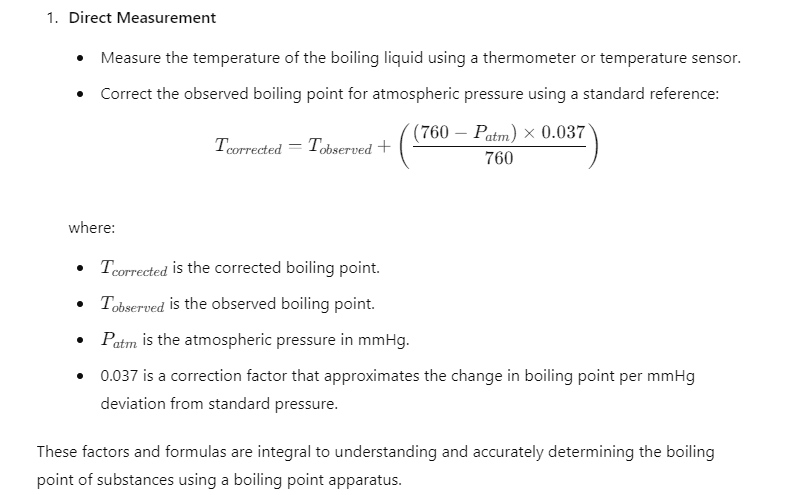
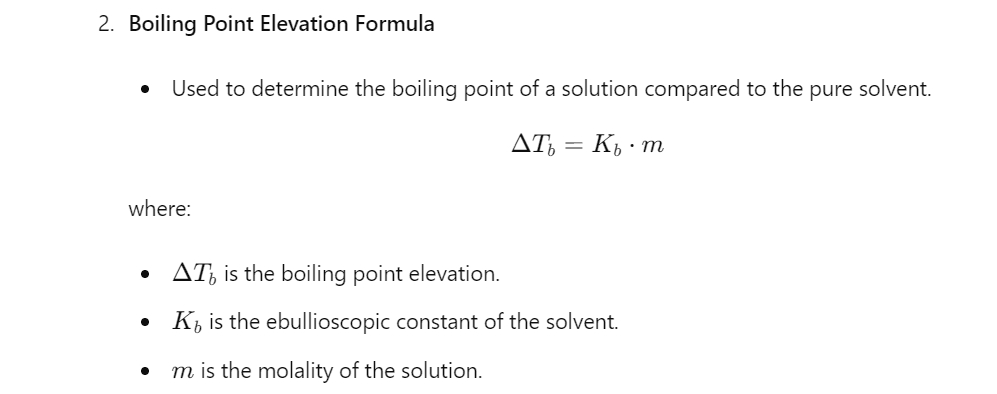
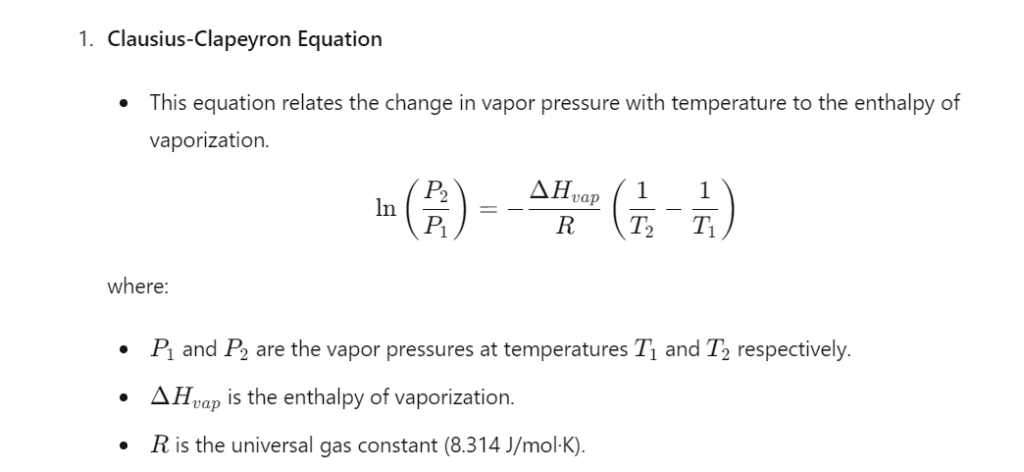
Applications of the Boiling Point Apparatus
Substance Identification
- Determining the boiling point helps in identifying unknown substances by comparing the observed boiling point with known values.
Purity Analysis
- Assessing the purity of a substance; pure substances have specific boiling points, while impurities typically lower the boiling point.
Chemical Synthesis
- Monitoring the boiling points of reactants and products in chemical synthesis to ensure correct processing and completion of reactions.
Quality Control
- Ensuring the quality and consistency of products in pharmaceutical, food, and chemical industries by verifying the boiling points of substances.
Distillation Process Optimization
- Aiding in the design and optimization of distillation processes by providing accurate boiling point data for various components.
Thermodynamic Studies
- Studying the thermodynamic properties of substances, including vapor pressure and enthalpy of vaporization.
Environmental Analysis
- Identifying and quantifying volatile organic compounds (VOCs) in environmental samples by their boiling points.
Forensic Science
- Analyzing chemical evidence in forensic investigations by determining the boiling points of substances found at crime scenes.
Educational Purposes
- Teaching students in chemistry and chemical engineering about phase transitions, boiling point determination, and related concepts.
Material Science
- Characterizing new materials and compounds by studying their boiling points to understand their thermal stability and behavior.
Pharmaceutical Research
- Determining the boiling points of new drug compounds and excipients to ensure proper formulation and storage conditions.
Petrochemical Industry
- Analyzing crude oil and its fractions by determining their boiling points, essential for refining and processing operations.
Cosmetics Industry
- Ensuring the stability and quality of volatile ingredients in cosmetics and personal care products by verifying their boiling points.
Flavor and Fragrance Industry
- Analyzing essential oils and aromatic compounds to determine their boiling points, crucial for product formulation and quality control.
Advantages and Disadvantages of the Boiling Point Apparatus
| Aspect | Advantages | Disadvantages |
| Accuracy | Provides precise measurement of boiling points, crucial for substance identification and purity analysis. | Calibration is required for accurate results, and improper calibration can lead to errors. |
| Versatility | Can be used for a wide range of substances, including volatile and non-volatile liquids. | Limited to substances that can be safely heated and boiled; not suitable for thermally unstable compounds. |
| Ease of Use | User-friendly setup with straightforward operation, making it accessible for both students and professionals. | Requires careful handling and setup to avoid accidents, especially with flammable or hazardous substances. |
| Control | Allows precise control over temperature and pressure, facilitating detailed studies of boiling behavior. | Manual control setups may require constant monitoring, leading to potential user fatigue. |
| Safety Features | Includes safety features like overheat protection and thermal cut-off, enhancing laboratory safety. | Despite safety features, risks of burns and exposure to vapors remain, necessitating strict safety protocols. |
| Durability | Constructed from high-quality, heat-resistant materials, ensuring long-term reliability and durability. | Glass components like the boiling flask and condenser are fragile and can break if mishandled. |
| Cost | Generally affordable and cost-effective for educational and professional laboratory settings. | High-end or specialized models can be expensive, potentially straining budget-constrained laboratories. |
| Speed | Allows rapid determination of boiling points, facilitating quick experimental workflows. | Rapid heating can lead to bumping and splashing, which can disrupt experiments and pose safety hazards. |
| Portability | Compact and portable, can be easily moved and set up in different laboratory environments. | Some models may be bulky and heavy, limiting ease of transport and requiring dedicated bench space. |
| Maintenance | Low maintenance requirements, with simple cleaning and occasional calibration ensuring consistent performance. | Regular maintenance is essential to prevent contamination and ensure accurate measurements, which can be time-consuming. |
Frequently Asked Questions (FAQ’s):
What apparatus is used in the boiling point experiment?
- The apparatus typically used in a boiling point experiment includes a boiling flask, heating source (such as a Bunsen burner or electric heater), thermometer or temperature sensor, condenser, and sometimes a manometer.
What is the instrument for boiling?
- The instrument for boiling is generally a heating device like a Bunsen burner, electric heater, or hot plate.
Which instrument is used to determine the boiling point of liquids?
- A boiling point apparatus, which includes a boiling flask, thermometer, and heating source, is used to determine the boiling point of liquids.
What is a boiling apparatus?
- A boiling apparatus is a setup used to determine the boiling point of a liquid, consisting of a boiling flask, heating source, thermometer, and sometimes additional components like a condenser.
What is true boiling point apparatus?
- True boiling point apparatus is used to measure the boiling point of a liquid accurately, typically under standardized conditions, to ensure precision in identifying the temperature at which a liquid boils under specific atmospheric pressure.
What tool is used for boiling?
- Common tools for boiling include Bunsen burners, electric heaters, and hot plates.
What apparatus is used to boil water?
- To boil water, one can use a pot or kettle on a stove, or in a laboratory setting, a boiling flask with a Bunsen burner or electric heater.
What tools are used to measure boiling point?
- Tools used to measure boiling point include a boiling flask, thermometer, heating source, and sometimes a condenser and manometer.
Why is boiling point used?
- Boiling point is used to identify and characterize substances, understand their purity, and study their physical and chemical properties.
What is boiling point principle?
- The boiling point principle states that the boiling point is the temperature at which the vapor pressure of a liquid equals the atmospheric pressure surrounding it.
What is boiling point in lab?
- In the lab, the boiling point is the temperature at which a liquid transforms into vapor under controlled conditions, typically measured using a boiling point apparatus.
How to identify boiling point?
- The boiling point is identified by gradually heating the liquid and noting the temperature at which it transitions to the vapor phase, indicated by the formation of steady bubbles.
How to calculate boiling point?
- Boiling point is generally measured directly using a thermometer in a controlled experimental setup. Corrections may be applied based on atmospheric pressure variations.
What is the normal boiling point?
- The normal boiling point is the temperature at which a liquid boils under a standard atmospheric pressure of 1 atm (101.3 kPa).
What is boiling point with example?
- An example of boiling point is water boiling at 100°C (212°F) at sea level under standard atmospheric pressure.
What defines boiling point?
- Boiling point is defined as the temperature at which the vapor pressure of a liquid equals the surrounding atmospheric pressure, causing the liquid to transition to vapor.
What are the principles of boiling?
- The principles of boiling include the concepts of vapor pressure, atmospheric pressure, and heat energy required to transition from the liquid phase to the vapor phase.
What are the advantages of boiling point?
- Advantages of boiling point determination include identifying substance purity, characterizing chemical compounds, and providing essential data for chemical reactions and processes.
What are the factors affecting boiling point?
- Factors affecting boiling point include atmospheric pressure, intermolecular forces, and the presence of impurities in the liquid.
What is an example of boiling?
- An example of boiling is water boiling on a stove at 100°C (212°F) under standard atmospheric pressure.
What is the concept of boiling point diagram?
- A boiling point apparatus diagram illustrates the relationship between the boiling points of components in a mixture and their compositions, often used in distillation processes.
What is the application of boiling point determination?
- Applications include substance identification, quality control, and studying the physical properties of liquids in research and industrial processes.
What is the boiling point method?
- The boiling point method involves heating a liquid and measuring the temperature at which it transitions to vapor, using a boiling point apparatus.
What is the concept of boiling?
- Boiling is the process in which a liquid turns into vapor when its vapor pressure equals the surrounding atmospheric pressure, involving heat energy input.
What are three types of boiling?
- Three types of boiling are nucleate boiling, transition boiling, and film boiling.
What is boiling uses?
- Boiling is used for cooking, sterilization, purification of liquids, and various industrial processes.
Why is boiling called?
- Boiling is called so because it involves the rapid formation of bubbles and vapor as a liquid transitions to its gaseous state.
Is boiling water safe?
- Boiling water is generally safe and is used to kill pathogens, making it safe for drinking.
What type of heat is boiling?
- Boiling involves the transfer of heat energy to a liquid, causing it to change phase from liquid to gas.
What is boil apparatus?
- Boil apparatus typically refers to equipment used to heat and boil liquids, such as a Bunsen burner, boiling flask, and other related components.
Which laboratory apparatus is used for boiling?
- Laboratory boiling point apparatus for boiling includes boiling flasks, Bunsen burners, hot plates, and condensers.
What is the apparatus boiling tube?
- A boiling tube is a thick-walled, cylindrical tube used in laboratories to heat substances directly over a flame.
What temperature water boil?
- Water boils at 100°C (212°F) at standard atmospheric pressure (1 atm).
What equipment is used for boiling?
- Equipment for boiling includes pots, kettles, boiling flasks, Bunsen burners, hot plates, and electric heaters.
What is the true boiling point apparatus used for?
- The true boiling point apparatus is used to measure the boiling point of liquids accurately under controlled and standardized conditions.
What are the three types of boiling?
- The three types of boiling are nucleate boiling, transition boiling, and film boiling.
What is boiling and examples?
- Boiling is the process where a liquid changes to a gas at its boiling point. Examples include water boiling at 100°C and ethanol boiling at 78.37°C.
Which instrument is used for boiling point?
- The instrument used for boiling point determination is the boiling point apparatus, which includes components like a boiling flask, thermometer, and heating source.