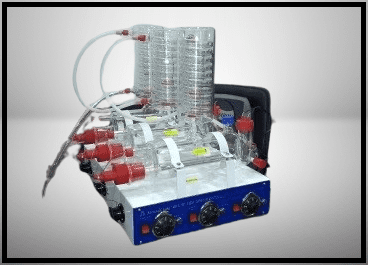
Double distillation unit In the realms of research and industrial Quality control laboratories, the quality of distilled water is paramount. The latest double distillation units are meticulously designed to meet the stringent demands of these environments, delivering water that is free from metallic ions and bacteria. These compact and efficient units incorporate advanced materials and technology to ensure unparalleled purity and reliability.
Superior Construction and Design:
The double distillation units are built with precision, featuring a high-purity boiler that ensures the production of contaminant-free water. The condenser, an equally critical component, is crafted from materials chosen for their resistance to corrosion and high temperatures, ensuring both durability and performance. The entire unit is supported by a powder-coated metal stand, which not only provides stability but also resists rust, ensuring a long operational life.
Working Principle of the Double Distillation Unit:
Double distillation units operate on a refined process designed to ensure the production of high-purity distilled water, free from contaminants such as metallic ions and bacteria. The working principle involves two consecutive stages of distillation, enhancing the purity of the water beyond what single distillation can achieve.
Stage 1: Primary Distillation
- Heating: The process begins with the heating of the source water in a high-purity boiler. The water is brought to a boil using an electric heater, converting it into steam.
- Vaporization: As the water reaches its boiling point, it starts to vaporize. This vaporization process helps to separate water molecules from impurities and contaminants that have higher boiling points, such as heavy metals and bacteria.
- Condensation: The steam then rises and enters the first condenser. Here, it cools down and condenses back into liquid form, producing the first batch of distilled water. This water is already of high purity, but to ensure even greater purity, it undergoes a second distillation process.
Stage 2: Secondary Distillation
- Re-boiling: The distilled water from the first stage is collected and then reintroduced into a secondary boiler. This boiler again heats the water until it reaches its boiling point, generating steam.
- Second Vaporization: During this second vaporization, any remaining impurities that might have passed through the first stage are further reduced. This double vaporization ensures that the distilled water is of the highest possible purity.
- Final Condensation: The steam produced in the secondary boiler moves into the second condenser, where it cools and condenses into highly purified distilled water.
Components and Functionality:
- Boilers: The primary and secondary boilers are constructed from high-purity materials to prevent contamination during the heating process.
- Condensers: Efficient coil condensers in both stages ensure effective cooling and condensation of the steam back into water.
- Insulation: Fiberglass insulated wiring and silicone rubber boots are used to resist high temperatures and ensure safety.
- Stand: The entire unit is mounted on a powder-coated metal stand, which provides durability and rust resistance.
Additional Safety and Operational Features:
- Distillation Apparatus Power Supply (DAPS): This feature ensures operational safety by automatically shutting off the heaters if the water level drops below the heating coils, preventing the heaters from burning out. Once the water level is restored, the heaters resume operation, allowing the distillation process to continue seamlessly.
- Soft Water Recommendation: To prevent scaling in the boilers and maintain the efficiency of the distillation process, the use of soft water is recommended.

Technical Specifications of the Double Distillation Unit:
| Specification | Details |
|---|---|
| Available Capacities | 1.5, 2.5, 3.5, 4, 5 Ltr/Hr |
| Input Voltage | 230 V AC ± 10, 50-60 Hz |
| Biological Activity | Pyrogen Free |
| pH Level | 6.9 – 7 |
| Conductivity | <1×10^-6 S/cm |
| Boiler Material | High purity material |
| Condenser Material | High purity material |
| Stand | Powder-coated metal for rust-free operation |
| Insulation | Fiberglass insulated wire, silicone rubber boot |
| Distillation Apparatus Power Supply (DAPS) | Automatic shutdown if water level falls below heating coil; automatic resumption when water level is restored |
| Additional Note | Use of soft water is recommended to prevent scaling and improve distillate quality |
Frequently Asked Questions:
What is the process of double distilling?
Answer: A Double distilling involves two consecutive distillation processes to achieve higher purity. The first distillation removes most impurities, and the second distillation further purifies the product.
- First Distillation:
- The mixture is heated, and the vapor is collected and condensed into a liquid.
- Second Distillation:
- The distillate from the first distillation is heated again, vaporized, and condensed, resulting in a higher purity product.
What is a double distillation unit in a laboratory?
Answer: A double distillation unit in a laboratory is equipment designed to perform the double distillation process, producing extremely pure distilled water. It typically consists of two boilers and two condensers arranged in series to facilitate two stages of distillation.
What is the process of double distillation of water?
The double distillation process of water involves:
- First Stage:
- Water is boiled, and the steam is condensed to remove initial impurities.
- Second Stage:
- The distilled water from the first stage is re-boiled, and the steam is condensed again, further purifying the water.
What is the principle of a double distillation unit?
Answer: The principle of a double distillation unit is to enhance the purity of distilled water by subjecting it to two separate distillation processes. This double distillation ensures the removal of even trace impurities and contaminants.
How does a distillation unit work?
Answer: A distillation unit works by:
- Heating: Boiling the mixture to vaporize the component with the lowest boiling point.
- Vaporization: Collecting the vapor and directing it to a condenser.
- Condensation: Cooling the vapor back into a liquid.
- Collection: Gathering the condensed liquid, now separated from the original mixture.
What is the function of double distilled water?
Answer: Double distilled water is used in applications requiring the highest purity levels, such as:
- Analytical and research laboratories
- Pharmaceutical preparations
- Chemical analysis
- Sensitive biological experiments
What is the water distillation principle?
Answer: The principle of water distillation is to separate water from its impurities by boiling and condensation. The water vaporizes, leaving impurities behind, and then condenses into pure water.
What is the pH of double distilled water?
The pH of double distilled water is typically neutral, around 6.9 to 7. However, it can slightly vary due to absorption of carbon dioxide from the air, which can lower the pH slightly.
What is the mechanism of distillation?
Answer: The mechanism of distillation involves:
- Boiling: Heating the liquid mixture to vaporize the component with the lowest boiling point.
- Vaporization: The vapor rises and is collected.
- Condensation: The vapor is cooled and condensed back into a liquid form.
- Collection: The purified liquid is collected separately.
What is the concept of distillation?
Answer: The concept of distillation is based on the separation of components in a mixture due to differences in their boiling points. By selectively boiling and condensing the components, they can be separated and purified.
What is the function of a distillation unit in a laboratory?
Answer: The function of a distillation unit in a laboratory is to purify liquids by separating them from impurities based on differences in boiling points. It is used for:
- Producing distilled water
- Purifying solvents
- Separating chemical mixtures
What is double effect distillation?
Answer: Double effect distillation is a process where two distillation stages are used, but the vapor from the first stage is used to heat the second stage. This improves energy efficiency by utilizing the latent heat from the first stage in the second stage.
What are the two main types of distillation?
Answer: Simple Distillation: Used for separating components with significantly different boiling points.
Fractional Distillation: Used for separating components with closer boiling points, utilizing a fractionating column to achieve higher separation efficiency.
What are the advantages of a distillation unit?
Answer: Advantages of a distillation unit include:
- High purity of the separated components
- Versatility in separating various mixtures
- Ability to remove both volatile and non-volatile impurities
- Reproducibility and reliability of the separation process
What is the principle of double distillation?
Answer: The principle of double distillation is to enhance the purity of a substance by subjecting it to two separate distillation processes. This removes a higher proportion of impurities and contaminants.
What are the applications of a distillation unit?
Answer: Applications of a distillation unit include:
- Producing pure water for laboratory and industrial use
- Separating and purifying chemicals
- Recovering solvents
- Refining crude oil
- Producing alcoholic beverages
What is the theory of distillation?
Answer: The theory of distillation is based on the equilibrium between liquid and vapor phases in a mixture. By heating the mixture, the component with the lower boiling point vaporizes first. This vapor can be condensed and collected, separating it from the other components.
What is the work of a distillation apparatus?
Answer: The work of a distillation apparatus is to:
- Heat the mixture to initiate boiling.
- Vaporize the component with the lower boiling point.
- Condense the vapor into a liquid.
- Collect the distilled liquid, now separated from impurities.
What is the working principle of continuous distillation?
Answer: The working principle of continuous distillation involves continuously feeding the mixture into the distillation column, where it is heated and vaporized. The vapor rises through the column, condensing at various points to separate components based on their boiling points. The process runs continuously, producing a constant output of purified components.
What is the temperature of distillation?
Answer: The temperature of distillation depends on the boiling points of the components in the mixture. For water, the boiling point is 100°C (212°F) at standard atmospheric pressure. In a distillation unit, the temperature is controlled to ensure efficient vaporization and condensation of the desired component.



The double distillation unit stands as a cornerstone in laboratory and industrial settings, ensuring purity and precision in extracting substances. An essential tool for achieving high-quality results in research and production processes.