A dynamic pass box is a small enclosure used in cleanroom environments to prevent contamination between two areas of differing cleanliness. It includes a fan or blower unit that provides air circulation and filtering to maintain a sterile environment during the transfer of materials or objects. Dynamic pass boxes are commonly used in pharmaceuticals, biotechnology, electronics, and food processing industries.
Dynamic pass box working principle
The working principle of a dynamic pass box involves the transfer of materials or objects from one area to another without the need for personnel to enter the cleanroom. The pass box is a small enclosure with two doors that are interlocked to prevent both doors from being open at the same time.
When the operator places the material inside the dynamic pass box and closes the door, a fan or blower unit is activated. The fan circulates air from the clean area through a HEPA (High-Efficiency Particulate Air) filter, which removes any particles or contaminants from the air.
The filtered air is then blown into the dynamic pass box, creating positive pressure inside the box, which helps to prevent the entry of contaminants from the outside. The operator can open the second door of the pass box, which allows them to remove the material from the box and transfer it to the cleanroom without the risk of contaminating the environment.
After the transfer is complete, the operator can close the second door, and the fan continues to operate for a set period to ensure that any contaminants that may have been introduced during the transfer process are removed from the air. Once the set period has elapsed, the fan automatically switches off, and the dynamic pass box is ready for its next use.
Overall, the working principle of a dynamic pass box involves the use of a fan or blower unit to provide air circulation and filtering, creating positive pressure inside the box, and ensuring that any contaminants are removed from the air during the transfer process.
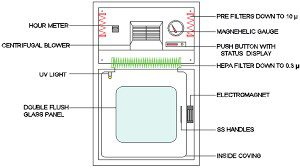
Types of Pass box
- Static Pass Box: A static pass box is a basic type of pass box that consists of two interlocked doors and is used for transferring materials or objects between cleanrooms or between cleanrooms and non-cleanroom areas. It does not have any built-in air filtration or sterilization system.
- Dynamic Pass Box: A dynamic pass box has a built-in air filtration system that maintains positive pressure within the pass box, preventing contaminants from entering the cleanroom environment. It is commonly used in pharmaceutical manufacturing to transfer materials or objects into or out of a cleanroom while maintaining the desired level of cleanliness.
- Air Shower Pass Box: An air shower pass box incorporates a high-velocity air shower to remove particles and contaminants from materials or objects before they are transferred into the cleanroom environment. It is commonly used in cleanrooms with high cleanliness requirements, such as those used in semiconductor or electronic manufacturing.
- UV Pass Box: A UV pass box uses UV-C light to sterilize materials or objects before they are transferred into the cleanroom environment. This type of pass box is commonly used in sterile pharmaceutical manufacturing.
- Rapid Transfer Port (RTP): An RTP is a type of pass box that uses a sealing mechanism to transfer materials or objects between two areas of differing cleanliness. It is commonly used in aseptic processing to maintain sterility.
- Material Dispensing Pass Box: This type of pass box is used for dispensing materials from one area to another. It is commonly used in pharmaceutical manufacturing to dispense raw materials or excipients into a process.
The selection of the appropriate type of pass box will depend on the specific requirements of the pharmaceutical process and the cleanroom environment in which it will be used. It is important to consult with cleanroom experts and follow regulatory guidelines when selecting and using pass boxes in pharmaceuticals.
Major Components of the Pass boxes.
| Component | Function |
|---|---|
| Enclosure | Provides a barrier between two areas of differing cleanliness, preventing the entry of contaminants from the outside. |
| Interlocked doors | Ensures that only one door can be opened at a time, preventing the simultaneous opening of both doors and maintaining the integrity of the clean environment. |
| HEPA filter | Filters the air that is circulated through the pass box, removing any particles or contaminants that may be present. |
| Fan or blower unit | Provides air circulation and ensures that filtered air is blown into the dynamic pass box, creating positive pressure that helps to prevent the entry of contaminants from the outside. |
| Control panel | Contains the controls for the fan, interlocking system, and other functions of the dynamic pass box, allowing operators to operate and monitor the pass box. |
| Lighting | Provides adequate illumination inside the pass box to allow operators to inspect and transfer materials safely. |
| Pressure gauge | Measures the positive pressure inside the dynamic pass box to ensure that the box is functioning correctly. |
| Sensors | Detects any anomalies or malfunctions in the pass box’s functions and alerts operators to take corrective action. |
Dynamic pass box differential pressure limit
The differential pressure limit for a dynamic pass box in a cleanroom environment depends on the specific requirements of the cleanroom and the type of operations being performed. In general, a dynamic pass box should maintain a positive pressure inside the box to prevent the entry of contaminants from the outside environment.
A positive pressure differential of 10-15 Pa (Pascal) is commonly recommended for dynamic pass boxes used in cleanrooms. This means that the pressure inside the pass box should be 10-15 Pa greater than the pressure outside the box.
However, the specific differential pressure limit may vary depending on the classification of the cleanroom and the type of operations being performed. For example, a sterile pharmaceutical manufacturing cleanroom may require a higher positive pressure differential than a non-sterile packaging cleanroom.
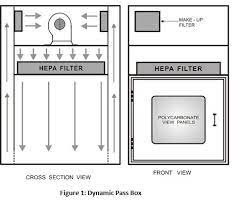
It is important to consult with cleanroom experts and follow the guidelines set forth by regulatory agencies to determine the appropriate differential pressure limit for a dynamic pass box in a cleanroom environment. Regular monitoring and maintenance of the pass box are also important to ensure that it continues to function within the specified pressure limits.
Validation of pass boxes in pharmaceuticals
| Validation Parameter | Description |
|---|---|
| Design Qualification (DQ) | A document that ensures the pass box is designed to meet the user requirements, including specifications for materials, dimensions, and functionality. |
| Installation Qualification (IQ) | A process that ensures the pass box is installed correctly, with proper placement, connections, and functionality. |
| Operational Qualification (OQ) | A process that ensures the pass box functions according to its design specifications, including testing of pressure differentials, airflow velocity, and HEPA filter efficiency. |
| Performance Qualification (PQ) | A process that ensures the pass box performs as intended in a real-world setting, including testing of microbiological contamination levels and particle counts. |
| Cleanroom Classification | Pass boxes should be installed in cleanrooms with the appropriate classification based on their intended use, such as ISO Class 5 for aseptic processing or ISO Class 7 for non-sterile manufacturing. |
| Material Compatibility | Pass boxes should be constructed of materials that are compatible with the materials being transferred, such as stainless steel or other non-porous materials. |
| Airflow Velocity | Pass boxes should have proper airflow velocity to prevent contamination from entering or leaving the cleanroom environment. |
| Pressure Differential | Pass boxes should have proper pressure differentials to maintain the desired direction of airflow and prevent cross-contamination between areas of different cleanliness. |
| HEPA Filter Efficiency | Pass boxes should have HEPA filters with appropriate efficiency to ensure that the air entering the cleanroom is free of particulate and microbial contamination. |
| Sterility Assurance | Pass boxes used in aseptic processing should be validated for sterility assurance, including testing for bacterial endotoxins and bioburden. |
| Maintenance and Monitoring | Pass boxes should be regularly maintained and monitored to ensure continued functionality and compliance with regulatory requirements. |

Applications of Pass box in Pharma.
| Application | Description |
|---|---|
| Material transfer | Pass boxes are commonly used to transfer materials or objects between two areas of differing cleanliness without the need for personnel to enter the cleanroom, preventing the risk of contamination. This is particularly important in pharmaceutical plants where the introduction of contaminants can compromise the quality of the products being manufactured. |
| Equipment transfer | Pass boxes can also be used to transfer equipment or components between different cleanrooms or between cleanrooms and non-cleanroom areas. This helps to prevent the introduction of contaminants into the cleanroom environment and ensures that the equipment or components being transferred remain sterile. |
| Waste transfer | Pass boxes can also be used to transfer waste materials from the cleanroom to non-cleanroom areas. The pass box ensures that the waste materials are contained and do not contaminate the cleanroom environment. |
| Product sampling | Pass boxes can be used to transfer product samples from the cleanroom to a testing laboratory for analysis. The pass box ensures that the samples remain sterile and that the laboratory environment is not contaminated. |
| Material storage | Pass boxes can be used as temporary storage areas for materials or components before they are transferred into the cleanroom environment. The pass box ensures that the materials remain sterile while they are stored and prevents the introduction of contaminants into the cleanroom environment. |
Frequently Asked Questions Dynamic Pass Box
What is a pass box?
Answer: A pass box is a device used to transfer materials or objects between two areas of differing cleanliness, typically within a cleanroom environment.
What is the purpose of a pass box in a pharmaceutical setting?
Answer: The purpose of a pass box in a pharmaceutical setting is to transfer materials or objects between cleanrooms or between cleanrooms and non-cleanroom areas while maintaining the integrity of the cleanroom environment.
What are the components of a pass box?
Answer: The components of a pass box typically include an enclosure, interlocked doors, HEPA filters, a fan or blower unit, a control panel, lighting, a pressure gauge, and sensors.
How does a pass box work?

Answer: A pass box works by using a positive pressure differential to prevent the entry of contaminants into the cleanroom environment. Materials or objects are placed into the pass box and then transferred through interlocked doors to the other side of the Dynamic Pass Box.
What is the importance of HEPA filters in a pass box?
Answer: HEPA filters are important in a pass box because they remove particles and contaminants from the air that is circulated through the Dynamic Pass Box, helping to maintain the cleanliness of the environment.
How often should HEPA filters be replaced in a pass box?
Answer: HEPA filters should be replaced regularly according to the manufacturer’s instructions and the specific requirements of the cleanroom.
What is the purpose of interlocked doors in a pass box?
Answer: Interlocked doors prevent the simultaneous opening of both doors, maintaining the integrity of the cleanroom environment and preventing the entry of contaminants.
What is the importance of maintaining positive pressure in a pass box?
Answer: Maintaining positive pressure in a Dynamic Pass Box is important to prevent the entry of contaminants from the outside environment and to maintain the cleanliness of the environment.
What is the ideal positive pressure differential for a pass box in a pharmaceutical setting?
Answer: The ideal positive pressure differential for a pass box in a pharmaceutical setting is typically 10-15 Pa greater than the pressure outside the box.
How should pass boxes be cleaned and disinfected?
Answer: Pass boxes should be cleaned and disinfected regularly according to the manufacturer’s instructions and the specific requirements of the cleanroom.
What are the safety considerations when using a pass box?
Answer: Safety considerations when using a pass box include ensuring that personnel are trained in the proper use of the pass box, following the manufacturer’s instructions for operation, and regularly inspecting and maintaining the pass box to ensure that it is functioning correctly.
What are the types of pass boxes available for use in pharmaceuticals?
Answer: The types of pass boxes available for use in pharmaceuticals include standard pass boxes, air shower pass boxes, and UV pass boxes.
What is an air shower pass box?
Answer: An air shower pass box is a type of pass box that uses high-velocity air jets to remove particles and contaminants from materials or objects before they are transferred into the cleanroom environment.
What is a UV pass box?
Answer: A UV pass box is a type of pass box that uses UV-C light to sterilize materials or objects before they are transferred into the cleanroom environment.
What are the regulatory requirements for pass boxes in pharmaceuticals?
Answer: The regulatory requirements for pass boxes in pharmaceuticals vary depending on the country and region. It is important to consult with regulatory agencies and follow their guidelines for the use and maintenance of pass boxes in pharmaceuticals.
