Flame photometer, also known as flame emission spectroscopy, is a crucial analytical technique in the field of chemistry for detecting and quantifying metal ions, particularly those of alkali and alkaline earth metals. Developed in the 1980s by Bowling Barnes, David Richardson, John Berry, and Robert Hood, the flame photometer measures the light emitted by metals introduced into a flame. This article delves into the principle, components, and working procedure of the flame photometer, providing a comprehensive understanding of this essential instrument.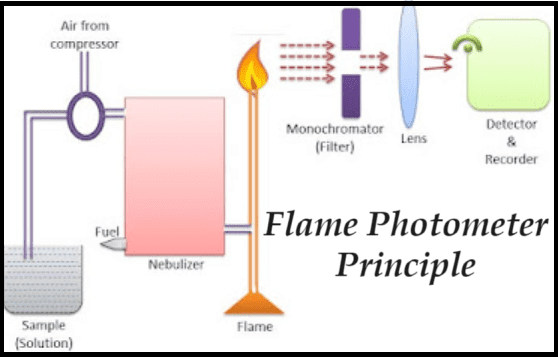
Flame Photometer Principle
The principle underlying flame photometry is based on the fact that when a metal is introduced into a flame, it emits light at a specific wavelength. This emission occurs because the compounds of alkali and alkaline earth metals dissociate into atoms within the flame. Some of these atoms get excited to higher energy levels due to the thermal energy of the flame. As these excited atoms are unstable at higher energy levels, they emit radiation as they return to their ground state. The emitted radiation, which generally lies within the visible spectrum, is characteristic of the specific metal present in the sample.
The intensity of the emitted light is directly proportional to the concentration of the metal in the sample. Thus, by measuring the intensity of the emitted light, the concentration of the metal ion can be determined. The relationship between the intensity of emitted light and the concentration of the element is described by the Scheibe-Lomakin equation:


Components of Flame Photometer
A simple flame photometer consists of the following key components:
1. Source of Flame
The burner, which acts as the source of the flame, is crucial for maintaining a constant temperature necessary for the analysis. The temperature of the flame significantly influences the accuracy of the measurements. Common types of burners used in flame photometers include:
- Total Consumption Burner: This burner aspirates the sample solution through a capillary due to the high pressure of fuel and oxidant, which are generally hydrogen and oxygen. While it ensures the entire consumption of the sample, it produces a non-uniform and turbulent flame.
- Premix Burner: In this type of burner, the sample, fuel, and oxidant are thoroughly mixed before being aspirated and reaching the flame. It produces a uniform flame, though there is a considerable loss of the sample (up to 95%).
2. Simple Colour Filters
Filters are essential for isolating the specific wavelength of the emission to be measured, thereby eliminating irrelevant emissions. A simple flame photometer contains a filter wheel with specific filters for different elements. When analyzing a particular element, the corresponding filter is selected.
3. Photo-detector
The intensity of the radiation emitted by the flame is measured by a photo-detector. Since the emitted radiation is primarily in the visible region, conventional detectors like photovoltaic cells or phototubes are used. Modern flame photometer often use photomultiplier tubes as detectors, providing electrical signals proportional to the intensity of the light.
Working Procedure of Flame Photometer:
The working procedure of a flame photometer involves several steps to ensure accurate measurements of metal ion concentrations:
- Preparation of Solutions: Both the standard stock solution and the sample solution are prepared using fresh distilled water.
- Flame Calibration: The flame of the photometer is calibrated by adjusting the air and gas, allowing it to stabilize for about five minutes.
- Instrument Setup: The instrument is switched on, and appropriate colour filters are inserted into the filter chamber. The galvanometer readings are adjusted to zero by spraying distilled water into the flame.
- Sensitivity Adjustment: The sensitivity of the instrument is adjusted by spraying the most concentrated standard working solution into the flame. The full-scale deflection of the galvanometer is recorded.
- Standard Solutions Analysis: Each standard working solution is sprayed into the flame three times, and the galvanometer readings are recorded. After each spray, the apparatus is thoroughly washed.
- Plotting the Calibration Curve: The mean of the galvanometer readings is calculated, and a graph of concentration against the galvanometer reading is plotted to determine the concentration of the element in the sample.
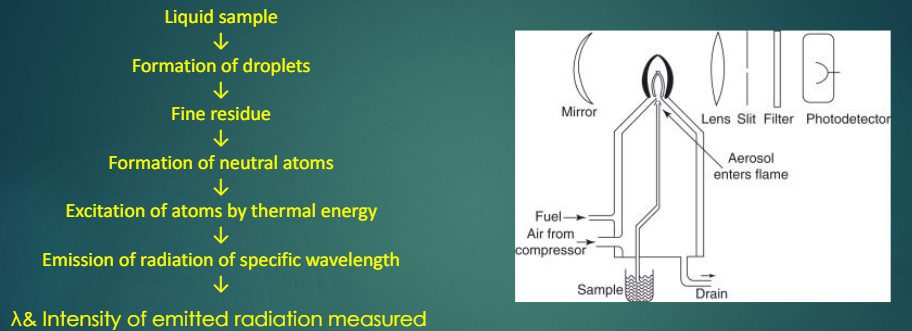
The analysis process involves several stages:
- Desolvation: Drying the sample solution in the flame, leading to the evaporation of the solvent and dehydration of metal particles.
- Vaporization: Further dehydration and evaporation of the metal particles.
- Atomization: Separation of all atoms in the chemical substance, reducing the metal ions to metal atoms by the flame.
- Excitation: Absorption of energy by the atoms, causing them to jump to a higher energy state.
- Emission: Emission of radiation with a characteristic wavelength as the atoms return to the ground state, measured by the photo-detector.
Applications of Flame Photometer
Flame photometer is versatile, allowing for both qualitative and quantitative analysis of elements. Its applications span various fields:
- Soil Analysis: Determining the presence of alkali and alkaline earth metals in soil samples to guide the application of specific fertilizers.
- Biological Fluids: Measuring the concentrations of sodium (Na+^++) and potassium (K+^++) ions in blood serum, crucial for various metabolic functions.
- Beverage Analysis: Analyzing soft drinks, fruit juices, and alcoholic beverages to determine the concentrations of various metals and elements.
- Construction Materials: Determining the concentrations of calcium and magnesium in cement.
- Fuel Analysis: Detecting the presence of lead in petrol.
Advantages of Flame Photometer
Flame photometer offers several advantages:
- Simplicity and Economy: The method is straightforward and cost-effective.
- Quick and Convenient: Provides rapid and convenient analysis.
- Selective and Sensitive: Highly selective and sensitive, capable of detecting very low concentrations (parts per million to parts per billion).
- Qualitative and Quantitative: Suitable for both qualitative and quantitative analysis.
- Compensation for Interference: Compensates for unexpected interfering materials present in the sample solution.
- Rare Element Analysis: Can estimate elements that are rarely analyzed.
Disadvantages of Flame Photometer
Despite its advantages, flame photometer has some limitations:
- Accuracy Issues: The accurate concentration of metal ions in the solution cannot always be measured precisely.
- Inert Gases Detection: Cannot directly detect and determine the presence of inert gases.
- Molecular Structure Information: Does not provide information about the molecular structure of the metal present in the sample.
- Sample Preparation: Only liquid samples can be used, and sample preparation can be lengthy.
- Limited Element Detection: Not suitable for the direct determination of every metal atom. Elements like carbon, hydrogen, and halides cannot be detected due to their non-radiating nature.
Conclusion
Flame photometer remains an indispensable tool in analytical chemistry, enabling the detection and quantification of metal ions with high sensitivity and selectivity. By understanding its principle, components, and working procedure, scientists can leverage this technique for various applications, from soil and biological fluid analysis to beverage and construction material testing. Despite its limitations, the simplicity, cost-effectiveness, and versatility of flame photometer make it a valuable method for many analytical needs.
Frequently asked question (FAQ’s)
What is a flame photometer used for?
Answer: A flame photometer is used for the detection and quantification of metal ions, particularly alkali and alkaline earth metals like sodium (Na), potassium (K), lithium (Li), calcium (Ca), and cesium (Cs). It is widely used in:
- Clinical Laboratories: Measuring electrolyte levels in biological fluids such as blood serum.
- Agriculture: Determining soil nutrient content to guide fertilizer application.
- Beverage Industry: Analyzing the metal content in soft drinks, fruit juices, and alcoholic beverages.
- Cement Industry: Measuring calcium and magnesium concentrations.
- Environmental Monitoring: Detecting metal pollutants in water and other environmental samples.
What is the principle of photometry?
Answer: The principle of photometry is based on the measurement of light intensity. Photometry involves quantifying the amount of light that a sample absorbs or emits. In analytical applications, photometric measurements are used to determine the concentration of analytes in a sample, based on the Beer-Lambert law, which states that the absorbance is directly proportional to the concentration of the absorbing species.
What is the principle of flame spectroscopy?
Answer: The principle of flame spectroscopy, specifically flame photometer, is based on the excitation of metal ions by a flame. When a sample containing metal ions is introduced into the flame, the thermal energy excites the metal atoms, causing them to emit light at characteristic wavelengths as they return to their ground state. The intensity of this emitted light is proportional to the concentration of the metal ion in the sample.
What are the uses of flame?
Answer: Flames are used in various applications, including:
- Heating and Combustion: In industries and laboratories for heating substances.
- Soldering and Welding: In manufacturing and repair work.
- Cooking: In kitchens for food preparation.
- Light Source: In lamps and torches.
- Scientific Analysis: In techniques like flame photometer for analyzing metal ion concentrations.
What are the uses of flame test?
Answer: The flame test is used to identify the presence of certain metal ions based on the characteristic color they emit when heated in a flame. Applications include:
- Qualitative Analysis: Identifying metal ions in laboratory samples.
- Educational Demonstrations: Teaching about emission spectra and electron transitions.
- Forensics: Detecting metal residues at crime scenes.
- Geology: Identifying mineral composition.
- Quality Control: Verifying metal content in industrial processes.
What is the theory of flame photometry?
Answer: The theory of flame photometer is based on the excitation of metal atoms in a flame. When a sample containing metal ions is aspirated into a flame, the heat causes the metal atoms to excite and then emit light at characteristic wavelengths as they return to their ground state. The emitted light’s intensity is measured and is directly proportional to the concentration of the metal ions in the sample.
How is a photometer used?
Answer: A photometer is used by following these steps:
- Calibration: Calibrate the instrument using standard solutions of known concentrations.
- Sample Preparation: Prepare the sample and ensure it is in the proper form (liquid, solid, etc.).
- Measurement: Introduce the sample to the photometer. In flame photometry, this involves aspirating the sample into the flame.
- Data Collection: The photometer measures the intensity of light (emission or absorption) from the sample.
- Analysis: Use the calibration curve to determine the concentration of the analyte in the sample based on the measured intensity.
What are the applications of photometer?
Answer: Photometers are used in various applications, including:
- Environmental Monitoring: Measuring pollutant concentrations in air and water.
- Clinical Diagnostics: Analyzing blood, urine, and other biological fluids.
- Pharmaceuticals: Quality control of drugs and active ingredients.
- Food and Beverage Industry: Ensuring product safety and quality.
- Chemical Research: Studying reaction kinetics and properties of compounds.
Which gas is used in flame photometry?
Answer: Common gases used in flame photometry include:
- Fuel: Usually acetylene or propane.
- Oxidant: Typically air or oxygen.
Who invented the flame photometer?
Answer: The flame photometer was developed in the 1980s by Bowling Barnes, David Richardson, John Berry, and Robert Hood.
What is the working principle of flame test?
Answer: The working principle of the flame test is based on the excitation of electrons in metal ions by the heat of the flame. When a sample is introduced into the flame, the metal ions absorb energy and their electrons jump to higher energy levels. As the electrons return to their ground state, they emit light at characteristic wavelengths, which can be observed as distinct flame colors.
What is the law of photometry?
Answer: The primary law of photometry is the Beer-Lambert law, which states that the absorbance of light by a substance is directly proportional to its concentration and the path length of the light through the sample:

What is the unit of a photometer?
Answer: The unit of measurement in photometry is typically the lux (lx), which measures illuminance, or the amount of light per unit area. For flame photometry, the unit often used is relative intensity or concentration units like parts per million (ppm) or parts per billion (ppb).
What is the theory of photometry?
Answer: The theory of photometry involves the measurement of light intensity and its interaction with substances. It encompasses the principles of light absorption, emission, and transmission, and is used to quantify the concentration of analytes in a sample based on the Beer-Lambert law.
What color flame is NA?
Answer: The flame color for sodium (Na) is bright yellow.
What color flame is copper?
Answer: The flame color for copper (Cu) is blue-green.
What is the color of sodium?
Answer: The characteristic flame color of sodium is yellow.
What is the use of flame test?
Answer: The flame test is used to:
- Identify Metal Ions: Based on characteristic flame colors.
- Educational Purposes: Demonstrating electron transitions and emission spectra.
- Forensics: Detecting metallic residues.
- Mineral Identification: In geological studies.
- Quality Control: Verifying metal content in industrial samples.
What are the five applications of flame photometry?
Answer:
- Clinical Analysis: Measuring sodium and potassium levels in blood.
- Agricultural Testing: Determining soil nutrient levels.
- Beverage Industry: Analyzing metal content in drinks.
- Cement Industry: Measuring calcium and magnesium.
- Environmental Monitoring: Detecting metal pollutants in water.
What are the advantages of a photometer?
Answer:
- Simplicity: Easy to use and interpret results.
- Cost-Effective: Inexpensive compared to other analytical techniques.
- Sensitivity: Can detect low concentrations of analytes.
- Versatility: Used in various fields such as environmental monitoring, clinical diagnostics, and industrial quality control.
- Rapid Analysis: Provides quick results for real-time decision making.