Gas chromatography (GC) is a type of chromatography technique used to separate and analyze components of a mixture based on their volatilities and affinity for a stationary phase. It involves the vaporization of a sample and its passage through a column packed with a stationary phase, where the separation occurs based on differences in partitioning behavior between the mobile phase (a carrier gas) and the stationary phase.
how does gas chromatography work
In Gas chromatography (GC), a sample is introduced into the chromatograph, typically as a vapor or a gas. The sample is then carried by the flow of an inert gas, such as helium or nitrogen, through a column that contains the stationary phase. The stationary phase is a high-boiling liquid or solid adsorbent material that is coated on the surface of the column or packed inside the column. As the sample components interact with the stationary phase, they are separated based on their partitioning behavior, with components that have a higher affinity for the stationary phase taking longer to travel through the column compared to components with lower affinity.
The separated components of the sample are detected as they exit the column, typically using a detector that measures the concentration of the components. Common detectors used in Gas chromatography include flame ionization detector (FID), thermal conductivity detector (TCD), and mass spectrometer (MS), among others. The data generated from the detector is used to create a chromatogram, which is a graphical representation of the separated components, their retention times (time taken to elute from the column), and their relative concentrations. 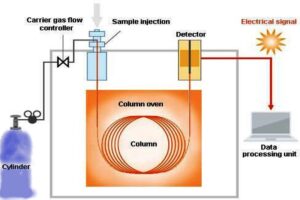 GC has a wide range of applications in various Quality Control Labs fields such as pharmaceuticals, environmental analysis, forensics, petrochemicals, food and beverage analysis, and many more. It is a powerful analytical technique that allows for the precise separation and quantification of components in complex mixtures.
GC has a wide range of applications in various Quality Control Labs fields such as pharmaceuticals, environmental analysis, forensics, petrochemicals, food and beverage analysis, and many more. It is a powerful analytical technique that allows for the precise separation and quantification of components in complex mixtures.
Gas Chromatography Machine and its Major Components:
The basic components of a Gas chromatography system include a sample injection port, a separation column, a detector, and a data recording system. Let’s delve into the structure and fundamentals of gas chromatography:
Sample Injection Port: The sample injection port is where the sample is introduced into the GC system. It may be a heated port or a split/splitless injection port. The sample is typically vaporized and injected into the carrier gas stream, which carries it into the separation column.
Separation Column: The separation column is a critical component of a GC system. It is typically a long, narrow tube packed with a stationary phase or coated with a stationary phase. The stationary phase is chosen based on its selectivity towards the components of the sample to be separated. As the sample components interact with the stationary phase, they are separated based on their partitioning behavior and elute from the column at different retention times.
Carrier Gas: The carrier gas is an inert gas, such as helium, nitrogen, or hydrogen, that flows through the separation column and carries the sample components along with it. The choice of carrier gas depends on factors such as the stationary phase, the sample composition, and the detector used.
Detector: The detector is used to detect the separated components as they elute from the separation column. There are various types of detectors used in GC, including flame ionization detector (FID), thermal conductivity detector (TCD), electron capture detector (ECD), and mass spectrometer (MS), among others. The choice of the detector depends on factors such as the analyte’s properties, sensitivity requirements, and the application of the GC analysis.
Data Recording System: The data recording system is used to capture and analyze the signals generated by the detector. It may be a chart recorder, a data acquisition system, or a computer-based system that records and analyzes the detector output to generate a chromatogram, which is a graphical representation of the separated components.
Gas chromatography principle:
The fundamental principle of GC is based on the partitioning behavior of sample components between the mobile phase (carrier gas) and the stationary phase. The sample components with higher affinity for the stationary phase will spend more time interacting with the stationary phase, resulting in longer retention times, while components with lower affinity for the stationary phase will elute from the column faster, resulting in shorter retention times. The separation of components is based on their differential partitioning behavior, and the resulting chromatogram provides information about the identity, concentration, and retention time of the components in the sample.
Principle of gas chromatography:
GC is a powerful analytical technique that offers high resolution, sensitivity, and selectivity for the separation and analysis of volatile and semi-volatile compounds in complex mixtures. It is widely used in various applications across fields such as pharmaceuticals, environmental analysis, forensics, petrochemicals, food and beverage analysis, and many more.
Retention time gas chromatography
Retention time in GC refers to the time it takes for an analyte to pass through the chromatographic column and reach the detector after it has been injected into the column. It is an important parameter used to identify and quantify analytes in a GC analysis. The retention time is influenced by various factors, such as the type of stationary phase in the column, the mobile phase (carrier gas) flow rate, the column temperature, and the chemical properties of the analyte. Retention time is typically reported in minutes and is used as a characteristic feature to identify analytes by comparing their retention times to those of known standards or reference compounds. Retention time can be used as a qualitative identification tool, as well as for quantitative analysis by measuring the area or height of the chromatographic peak at the retention time of the analyte of interest. It is important to establish retention time reproducibility in GC analysis through proper method development, optimization, and validation to ensure accurate and reliable results.
Applications of Gas chromatography (GC)
GC is a versatile analytical technique with a wide range of applications in various fields. Some of the common applications of GC include:
Environmental Analysis: GC is used for analyzing environmental samples to detect and quantify pollutants, such as volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs), pesticides, and other contaminants in air, water, soil, and sediment samples. GC is also used for monitoring emissions from industrial processes and evaluating the effectiveness of environmental remediation efforts.
Pharmaceuticals: GC is widely used in the pharmaceutical industry for drug discovery, development, and quality control. It is employed for analyzing drug formulations, determining drug purity, identifying impurities, and studying drug metabolism. GC is also used in forensic toxicology for analyzing drugs and their metabolites in biological samples, such as blood, urine, and tissues.
Petrochemicals: GC plays a crucial role in the analysis of petrochemicals, including crude oil, gasoline, diesel, and other petroleum products. It is used for characterizing and quantifying the composition of hydrocarbons, determining the octane rating of gasoline, analyzing additives and contaminants in fuels, and monitoring process efficiency in refineries.
Food and Beverage Analysis: GC is used for analyzing food and beverage samples to determine the presence and concentration of flavor compounds, aroma compounds, pesticides, preservatives, and other contaminants. GC is also used for analyzing alcoholic beverages, such as beer, wine, and spirits, for determining their quality, authenticity, and compliance with regulatory standards.
Forensics: GC is employed in forensic science for analyzing volatile and semi-volatile compounds in evidence samples, such as arson residues, explosives, drugs, and toxicological samples. GC is used for identifying and quantifying trace evidence, analyzing fire debris, investigating drug-related crimes, and determining the presence of toxic substances in biological samples.
Environmental Science: GC is used in environmental science research for studying the fate and transport of pollutants in the environment, assessing air quality, and monitoring emissions from industrial processes. GC is also used for analyzing volatile organic compounds (VOCs) in ambient air, determining greenhouse gas concentrations, and studying biogenic emissions from plants and soils.
Flavor and Fragrance Industry: GC is widely used in the flavor and fragrance industry for analyzing complex mixtures of volatile compounds that contribute to the aroma and flavor of various products, such as perfumes, cosmetics, and food products. GC is used for characterizing and quantifying volatile flavor and fragrance compounds, optimizing formulations, and ensuring product quality and consistency.
Research and Academia: GC is extensively used in research and academic settings for a wide range of applications, including environmental studies, chemical analysis, materials science, natural product analysis, and bioanalytical research. GC is employed for identifying and quantifying volatile and semi-volatile compounds in diverse sample matrices, enabling scientists to study and understand the composition and behavior of complex mixtures.
These are just a few examples of the numerous applications of gas chromatography in various fields. GC is a powerful and widely used analytical technique that offers high sensitivity, selectivity, and resolution for the separation and analysis of volatile and semi-volatile compounds in complex mixtures, making it an essential tool in many scientific and industrial applications.
Different injection methods in gas Chromatography
In gas chromatography (GC), there are several different methods for injecting a sample into the chromatographic system. The choice of injection method depends on the type of sample, the desired sensitivity, and the chromatographic system being used. The main injection methods in GC are split injection, splitless injection, direct injection, and on-column injection. Let’s briefly explain each of these methods:
- Split Injection: In split injection, a portion of the sample is introduced into the GC column while the remaining portion is directed to a vent or waste, hence the term “split.” This method is commonly used when the sample is complex or contains high concentrations of analytes. The sample is typically injected into a heated injection port where it rapidly vaporizes and is carried onto the GC column by the flow of carrier gas. The split ratio, which determines the amount of sample that goes to the column versus the vent, can be adjusted to control the sensitivity and prevent column overload.
- Splitless Injection: In splitless injection, the entire sample is introduced into the GC column without splitting, resulting in a higher sensitivity compared to split injection. This method is commonly used for samples with low analyte concentrations or when maximum sensitivity is desired. During splitless injection, the sample is introduced into the heated injection port and allowed to vaporize completely before the split vent is closed, directing all of the sample onto the column. This method typically results in higher analyte recovery and improved detection limits compared to split injection.
- Direct Injection: In direct injection, a small volume of the sample is injected directly into the GC column without passing through the heated injection port. This method is commonly used for samples that are already in the gas or vapor phase, such as headspace analysis, where volatile compounds are extracted from a sample matrix (e.g., solid, liquid) into a headspace and then directly injected into the column. Direct injection can also be used for gas samples or when a sample matrix is compatible with direct injection without causing contamination or column damage.
- On-column Injection: In on-column injection, the sample is introduced directly onto the head of the GC column without passing through an injection port or any other interface. This method is typically used for very small sample volumes or for samples that are already in the liquid or gas phase, such as capillary GC analysis of liquid samples. The on-column injection is considered a highly effective method for minimizing sample discrimination and improving analyte recovery, but it may require special column designs and is typically not used for routine GC analysis.
Each of these injection methods has its advantages and limitations, and the choice of method depends on the specific requirements of the analysis and the characteristics of the sample being analyzed. Factors such as sample concentration, sample matrix, analyte volatility, desired sensitivity, and column compatibility should be considered when selecting the appropriate injection method for a particular GC analysis. Proper optimization of the injection method is crucial for achieving reliable and accurate GC results.
Type of Samples for Gas Chromatography (GC) Analysis
Gas chromatography (GC) is a versatile technique that can be used for the analysis of a wide range of samples. Some common types of samples that can be analyzed using GC include:
- Volatile organic compounds (VOCs): GC is often used for the analysis of VOCs, which are organic compounds that have low boiling points and readily vaporize at room temperature. Examples of VOCs include solvents, fuels, flavors, fragrances, and environmental pollutants.
- Semi-volatile organic compounds (SVOCs): GC can also be used for the analysis of SVOCs, which are organic compounds that have higher boiling points and require higher temperatures for vaporization. Examples of SVOCs include pesticides, plasticizers, and pharmaceuticals.
- Polycyclic aromatic hydrocarbons (PAHs): GC is commonly used for the analysis of PAHs, which are a group of organic compounds that are often found in environmental samples such as air, water, soil, and sediment. PAHs are typically analyzed using GC due to their relatively high boiling points and thermal stability.
- Essential oils: GC is frequently used for the analysis of essential oils, which are complex mixtures of volatile compounds extracted from plants. GC can provide detailed information on the composition of essential oils, which is important for quality control and characterization purposes.
- Environmental samples: GC is widely used for the analysis of environmental samples, such as air, water, soil, and sediment, for the determination of various organic pollutants, including volatile and semi-volatile compounds, pesticides, polychlorinated biphenyls (PCBs), and other contaminants.
- Food and flavor samples: GC is commonly used for the analysis of food and flavor samples to determine the composition of volatile compounds responsible for the aroma and flavor of food products, such as spices, fruits, beverages, and dairy products.
- Forensic samples: GC is used in forensic science for the analysis of various samples, such as drugs of abuse, explosives, fire debris, and toxicology samples, to determine the presence and concentration of relevant compounds.
These are just a few examples of the types of samples that can be analyzed using GC. GC is a versatile technique that can be applied to a wide range of sample types in various industries and fields for qualitative and quantitative analysis of organic compounds. Proper sample preparation techniques and optimization of GC methods are essential for obtaining reliable and accurate results.
Frequently Asked Question about Gas Chromatography
What is Gas Chromatography (GC)?
Answer: Gas Chromatography (GC) is an analytical technique used to separate, identify, and quantify volatile and semi-volatile compounds in a sample based on their differential partitioning between a stationary phase and a mobile phase in a chromatographic column.
What are the applications of GC?
Answer: GC has a wide range of applications in various fields, including pharmaceuticals, environmental analysis, food and beverage analysis, forensic science, petrochemicals, flavors and fragrances, and many more.
How does GC work?
Answer: GC works by injecting a sample into a heated inlet, where it is vaporized and carried by a carrier gas through a chromatographic column. The analytes in the sample partition between the stationary phase in the column and the mobile phase (carrier gas), leading to their separation based on their different affinities to the stationary phase.
What are the different types of detectors used in GC?
Answer: Common types of detectors used in GC include
- Flame Ionization Detector (FID),
- Thermal Conductivity Detector (TCD),
- Electron Capture Detector (ECD),
- Mass Spectrometry (MS), and many others.
What are the factors that affect the resolution in GC?
Answer: Factors that affect the resolution in GC include column selectivity, column temperature, flow rate of the carrier gas, column dimensions, sample size, and injection technique.
What are the different injection methods used in GC?
Answer: Different injection methods used in GC include split injection, splitless injection, direct injection, and on-column injection, each with its advantages and limitations.
What are the common problems in GC analysis?
Answer: Common problems in GC analysis include contamination, baseline noise, peak tailing or fronting, poor resolution, column degradation, calibration issues, and others.
How can column selection impact GC analysis?
Answer: Column selection plays a critical role in GC analysis, as it determines the separation and resolution of analytes. Factors such as stationary phase, column length, column diameter, and film thickness need to be carefully considered for optimal results.
What is method validation in GC analysis?
Answer: Method validation is the process of evaluating and establishing the performance characteristics of a GC method to ensure its suitability for a specific analytical purpose. It involves assessing parameters such as accuracy, precision, linearity, selectivity, and robustness.
What are the advantages of GC as an analytical technique?
Answer: Some of the advantages of GC include high sensitivity, wide applicability, relatively fast analysis time, and the ability to analyze volatile and semi-volatile compounds. GC is also a highly precise and reliable technique when properly validated and optimized.
Gas Chromatography (Chrom-Ed Book Series)
For More information about Gas chromatography please comment below.

![Gas Chromatography (Chrom-Ed Book Series) by [Raymond P W Scott]](https://m.media-amazon.com/images/I/410-+DCKM0L.jpg)