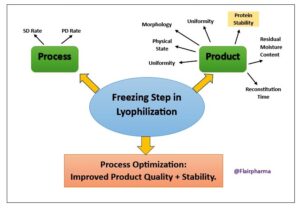
Importance of the Lyophilization Process in the pharmaceuticals
| Importance of Lyophilization in Pharmaceuticals |
|---|
| 1. Enhanced Stability: Lyophilization helps to improve the stability of pharmaceutical products by removing water, which is a critical factor in chemical degradation and microbial growth. |
| 2. Extended Shelf Life: The removal of water through lyophilization significantly extends the shelf life of pharmaceuticals, allowing for longer storage and transportation without degradation. |
| 3. Preservation of Bioactivity: Lyophilization preserves the bioactivity of sensitive drugs and biological materials by minimizing exposure to high temperatures, which can lead to denaturation or degradation. |
| 4. Convenient Reconstitution: Lyophilized products can be easily reconstituted by adding a suitable solvent, allowing for convenient administration, especially for injectable drugs and vaccines. |
| 5. Reduction of Product Weight and Volume: Lyophilization reduces the weight and volume of pharmaceutical products by removing water, making them more cost-effective to store, transport, and handle. |
| 6. Enhanced Solubility: Some lyophilized drugs exhibit improved solubility, which can enhance their bioavailability and therapeutic effectiveness in the body. |
| 7. Minimization of Chemical Reactions: Lyophilization minimizes the occurrence of chemical reactions between drug substances and excipients, leading to better product quality and consistency. |
| 8. Facilitation of Long-Term Storage: Lyophilized pharmaceuticals can be stored for longer periods at controlled temperatures, enabling stockpiling for emergencies or ensuring availability in remote locations. |
| 9. Reducing Cold Chain Dependencies: Lyophilization reduces the dependency on continuous cold chain storage and transportation, making it more feasible to reach areas with limited access to refrigeration facilities. |
| 10. Improved Patient Compliance: Lyophilized drugs can offer convenience to patients, as they often have longer expiration dates and are available in easy-to-use forms, leading to improved medication adherence. |
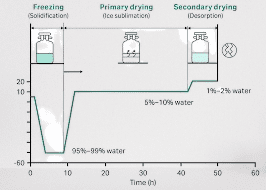
Lyophilization temperature
The temperature used in the lyophilization process can vary depending on the specific materials being processed. However, the general temperature range for lyophilization typically falls between -40°C to -50°C (-40°F to -58°F) during the freezing phase and around 0°C to -20°C (32°F to -4°F) during the primary drying phase.
Freezing Phase: The first step in lyophilization is freezing the material. The temperature is lowered to around -40°C to -50°C (-40°F to -58°F) to solidify the water or solvent in the product. This freezing process helps convert the liquid phase into a solid phase.
Primary Drying Phase: After the material is frozen, the vacuum is applied, and heat is introduced to initiate sublimation, where the frozen water or solvent transitions directly from a solid to a gas without passing through the liquid phase. The temperature is maintained at around 0°C to -20°C (32°F to -4°F) during this primary drying phase.
Secondary Drying Phase: Following the primary drying phase, some residual moisture may still be present in the material. In the secondary drying phase, the temperature is increased slightly, usually around 20°C to 40°C (68°F to 104°F), to facilitate the removal of any remaining moisture through desorption.
It’s important to note that the exact temperature and duration of each phase can vary depending on factors such as the specific product being lyophilized, the formulation, and the equipment being used. Process parameters are carefully optimized to ensure effective drying while preserving the integrity and stability of the product.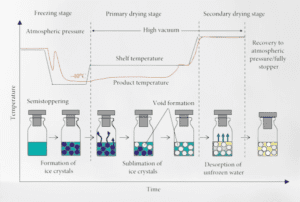
Lyophilization process flow chart
- Preparation and Loading
- ↓
- Pre-Freezing
- ↓
- Primary Drying (Sublimation)
- ↓
- Secondary Drying (Desorption)
- ↓
- End of Drying
- ↓
- Stoppering and Sealing
- ↓
- Product Removal
Please note that this flow chart provides a high-level overview of the lyophilization process. Each step can involve various sub-steps and considerations. Additionally, there may be additional steps involved in specific lyophilization processes based on the nature of the product and its formulation.
Advantages of lyophilization
Lyophilization, or freeze-drying, offers several advantages in various industries, including pharmaceuticals. Here are some key advantages of the lyophilization process:
- Enhanced Stability: Lyophilization improves the stability of sensitive materials by removing water or solvents, which are prone to degradation and can cause chemical reactions or microbial growth. This stabilization helps to maintain the integrity and potency of pharmaceutical products.
- Extended Shelf Life: By removing water, lyophilization significantly extends the shelf life of pharmaceuticals. The absence of water reduces the chance of degradation, allowing for longer storage and transportation without compromising product quality.
- Preservation of Bioactivity: Lyophilization is particularly beneficial for preserving the bioactivity of sensitive drugs, proteins, enzymes, and other biological materials. The process minimizes exposure to high temperatures that can denature or degrade these substances.
- Improved Solubility: Some lyophilized drugs exhibit improved solubility compared to their non-lyophilized counterparts. This enhanced solubility can enhance the drug’s bioavailability, leading to better therapeutic effects in the body.
- Convenient Reconstitution: Lyophilized products can be easily reconstituted by adding a suitable solvent, such as water. This convenience allows for easy administration, especially for injectable drugs and vaccines, reducing the preparation time and improving patient compliance.
- Reduced Weight and Volume: Lyophilization removes water, reducing the weight and volume of the product. This reduction makes the products more cost-effective to store, transport, and handle, as smaller volumes are required, resulting in lower shipping costs and storage space requirements.
- Minimized Chemical Reactions: Lyophilization reduces the occurrence of chemical reactions between drug substances and excipients. By removing water, the chances of interaction and degradation are reduced, resulting in better product quality and consistency.
- Facilitation of Long-Term Storage: Lyophilized pharmaceuticals can be stored for extended periods at controlled temperatures. This facilitates long-term storage, allowing for stockpiling for emergencies or ensuring availability in remote locations where refrigeration facilities may be limited.
- Versatility and Diverse Applications: Lyophilization can be applied to various pharmaceutical forms, including powders, tablets, vaccines, and injectables. Its versatility allows for preserving a wide range of drugs and biological materials.
- Reduced Cold Chain Dependencies: Lyophilized products are less dependent on continuous cold chain storage and transportation. They are more stable at higher temperatures, reducing the need for strict temperature control during distribution and making it more feasible to reach areas with limited refrigeration facilities.
These advantages highlight the importance and broad application of lyophilization in the pharmaceutical industry, offering improved stability, extended shelf life, and convenient handling of sensitive drugs and biological materials.
Frequently Asked Questions
What is the purpose of the pre-freezing step in the lyophilization process?
Answer: The pre-freezing step is performed to solidify the product before subjecting it to the vacuum. It helps to facilitate the subsequent sublimation of the frozen water or solvent during the primary drying phase.
What is the role of a condenser in the lyophilization process?
Answer: The condenser is responsible for collecting the water vapor that sublimates from the frozen product during the primary drying phase. It prevents the vapor from escaping into the vacuum and condenses it back into a solid or liquid form for removal.
Why is it important to achieve uniform freezing during the lyophilization process?
Answer: Uniform freezing ensures consistent ice crystal formation throughout the product. This promotes uniform sublimation and drying, leading to a more uniform and stable final product.
What is the purpose of the secondary drying phase in lyophilization?
Answer: The secondary drying phase aims to remove any residual moisture from the product. It involves slightly increasing the temperature to accelerate the desorption of the remaining water or solvent, ensuring the product is thoroughly dried.
How does the choice of lyoprotectant impact the lyophilization process?
Answer: Lyoprotectants are added to the product formulation to protect it from freeze-induced damage during freezing and drying. The choice of lyoprotectant can influence the stability, reconstitution properties, and overall success of the lyophilization process.
What is the impact of the chamber pressure on the lyophilization process?
Answer: The chamber pressure affects the rate of sublimation during primary drying. Lower pressures enhance sublimation, but excessively low pressures can lead to product collapse. Optimal chamber pressure is determined based on the specific product and process requirements.
What methods are commonly used for determining the endpoint of the drying process?
Answer: Common methods for determining the endpoint of the drying process include measuring the product’s residual moisture content, monitoring the rate of water vapor evolution, and assessing visual appearance and collapse characteristics.
How can the use of controlled nucleation techniques improve the lyophilization process?
Answer: Controlled nucleation techniques, such as ice fogging or nucleation-inducing substances, help promote uniform ice crystal formation and reduce product heterogeneity. This can result in improved product quality and drying efficiency.
What are the challenges associated with scaling up lyophilization processes from lab-scale to commercial production?
Answer: Scaling up lyophilization processes involves considerations such as heat transfer, equipment size, cycle time, and maintaining process uniformity. Achieving consistent product quality and maintaining control over process parameters can be challenging during scale-up.
How can process analytical technology (PAT) be applied to monitor and control the lyophilization process?
Answer: PAT techniques, such as near-infrared spectroscopy, thermogravimetric analysis, or impedance measurements, can be used to monitor critical process parameters and assess product quality in real-time. This enables better control and optimization of the lyophilization process.
You may also read about Sterility Test.

1 thought on “Lyophilization Process 2023”