The objective of this Standard Operating Procedure for Preparation numbering review issuance approval and control of layouts is to establish a consistent and organized process for the preparation, numbering, review, issuance, approval, and control of layouts to ensure accurate and standardized documentation across various projects or departments.
SOP for Preparation numbering review issuance approval and control of layouts.
- OBJECTIVE:
- To lay down the procedure for Preparation numbering review issuance approval and control of layouts.
- SCOPE:
- This SOP is applicable for all the layouts which are prepared.
- RESPONSIBILITY:
- Draughtsman – Engineering department shall be responsible for preparation of the layouts.
- Engineering – Head or designee shall be responsible for verifying the layouts for correctness.
- User Department – Head shall be responsible for checking the layouts.
- QA department shall be responsible for reviewing the layout and for assigning layout number.
- Head – QA (or) designee shall be responsible for reviewing and approving the layouts.
- ACCOUNTABILITY:
- Engineering – Head shall be accountable for layout preparation.
- DEFINITIONS:
- Preparation numbering review issuance approval and control of layouts.
- PROCEDURE:
- Layout shall be prepared for the following, but not limited to:
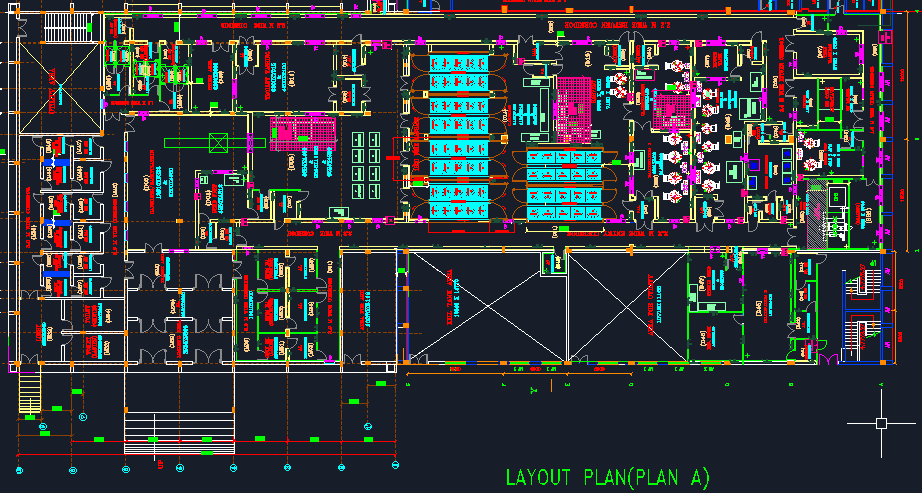
- Facility layout to include the following, but not limited to:
- Site Master Plan
- Man Material movement layout
- Drainage system
- Pest and Rodent control layout
- Equipment layout includes the following, but not limited to:
- As built drawing/ G A drawing
- Location of equipment in area (Equipment Layout)
- Schematic arrangement of the equipment/ P & I Drawing
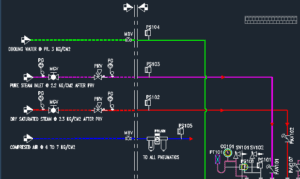
- Engineering/ Utility layouts includes the following, but not limited to:
- Schematic arrangement of AHU/ VU/ TFA and dust collector
- Duct layout, grill layout
- Pressure balancing
- Piping and Instrumentation diagram
- Layouts for Water system, Compressed air, Nitrogen, Chilled Water, Pure Steam and Plant Steam
- Preparation of layout:
- Engineering department shall prepare the draft copy of layout on a template for layouts as per Format no. SOP/EN/XXXYYY in consultation with user department.
- Layout depicting only the outline of facility, room shall indicate the scale and the unit.
- Any short form, diagrammatic representation, colour codes shall be explained in the layout.
- Engineering department shall put ‘DRAFT FOR REVIEW’ stamp in blue colour on the Layout and shall circulate the draft layout to the technical/user departments and QA for review, along with document review format i.e. Format no. SOP/QA/XXXYYY.
- Upon receipt of draft copy, the reviewers shall review and write their comments on the Document Review form and send to QA.
- QA document in-charge shall review the Layout for correctness and compliance.
- QA document in-charge shall assign a unique Layout number as per procedure mentioned in Step no. 6.5 and record the details in the Layout Numbering Record (Format No. SOP/EN/XXXYYY) and communicate the same to the Engineering Department through the Document Review Form.
- After incorporation of suggestion(s) as applicable, Engineering department shall take the final printout of the layout in A3 or A4 size paper and shall sign as ‘Prepared by’ and send it to User department for review and sign, along with draft layout and document review form.
- The User department – Head or designee shall sign as “Checked by” in the layout and send the documents to QA department for final approval from QA Head.
- QA – head or designee shall finally review and approved the layout by signing as “Approved by”.
- QA Document in-charge shall destroy the draft copy of layout and Document review form by shredding, after the final approval of the layout.
- QA Document in-charge shall then put a ‘Master Copy’ stamp in green ink, on the top right hand corner of the layout.
- The issuance of the layout shall be done as per procedure mentioned in step no. 6.3.
- Any additional copy (Photocopy or Original), if required shall be taken with prior authorization from Head – QA or designee.
- The layout prepared by external consultants shall be verified and shall be numbered as detail in section 6.5. Engineering department shall do the verification, which shall be checked by user department and approved by Head – QA.
- Issuance of layout:
- QA Document in-charge shall take photocopies from the master copy and stamped it as “Controlled Copy” with information of copy number, date of issue and issued by on bottom left hand corner with blue color ink.
- QA document in-charge shall issue the first control copy of any layout to the QA department for reference, second control copy to the Engineering department and third copy to the respective User department, if applicable.
- If need arise for an additional copy, concerned department head shall raise the Document Request (refer Format No. SOP/QA/XXXYYY); get it approved by Head – QA or designee.
- QA shall issue the control copy of the Layout as per procedure defined in SOP for Document Control, SOP/QA/XXXYYY
- Revision of layout:
- If the existing layout to be revised, due to any change/modification in the facility or system or equipment location, then User department shall raise a change control request as per change control procedure (refer SOP no. SOP/QA/XXXYYY).
- After approval of the change control, Layout shall be revised by the Engineering department and the changes shall be captured in the change history which shall be filed with change control document.
- The control, retrieval and destruction of the layouts shall be done by the procedure defined in SOP for Document Control, SOP/QA/XXXYYY
- Numbering of layout:
- Engineering department shall identify each layout with a unique number consisting of 15 characters.
- The numbering system for layouts shall follow the pattern – “LYT/ILS/X001-00”.
- The first 3 characters ‘LYT’ represent Layout.
- The fourth character ‘/’ represent a separator.
- 5th to 7th character ‘ILS’ represents .
- 8th character ‘/’ represent a separator.
- The 9th alphabetical character ‘X’ shall be as follows:
- ‘F’ for Facility Layout
- ‘E’ for Equipment layout and
- ‘U’ for Engineering/ Utility layout.
- 10th to 12th character stands for sequential drawing numbering starting from 001.
- 13th character is a hyphen as separator.
- The last two numerical characters (14th and 15th) are the version number of layout starting from 00 and continuing serially in increments of one unit.
- Note: There shall be separate series for each type of layout i.e. Facility, Equipment and Engineering.
- ABBREVIATIONS
- GA : General Arrangement
- CRF No. : Change Request Form number
- QA : Quality Assurance
- P & I : Process and Instrumentation
- REFERENCES
- Nil
- DISTRIBUTION LIST
- SOP shall be distributed to following departments Quality Assurance, Engineering as per user request.
- ANNEXURES
- Layout Numbering Record : SOP/EN/XXXYYY
- Template for layouts : SOP/EN/XXXYYY
LAYOUT NUMBERING RECORD
Preparation numbering review issuance approval and control of layouts
| S. No. | Title of the Layout | Layout Number | Allotted By (Sign & date) |
Preparation numbering review issuance approval and control of layouts
You may also read about Good Laboratory Practices (GLP)