Validation and Qualifications are the backbones of the pharma industry that has very critical impacts on the finished drug quality. Validation and Qualifications are applicable to every department and have Quality control, Microbiological Controls, Production process validation, Equipment Validation, cleaning validation, Clean room validation, Utility system validations, computer system Validation, and Qualifications.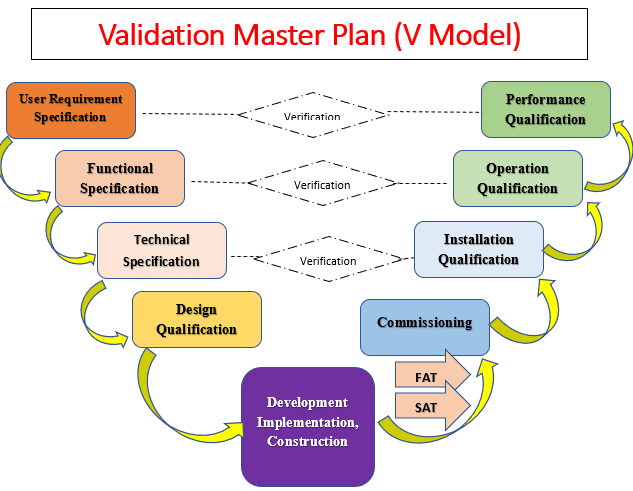
Do you want to know what is VALIDATION?
“Validation is defined as the gathering and evaluation of data, from the stage of process design to commercial production, which establishes scientific evidence that a process is capable of reliably delivering a quality product,” says the definition. proving that a process or system satisfies its predetermined requirements and quality standards.
According to the principles of good manufacturing practice (GMP), EU GMP is the “action of proving that any procedure, process, equipment, material, activity, or system actually leads to expected results.”
Validation is “creating a written proof that offers a high degree of assurance that a particular process will consistently produce a product fulfilling its pre-determined specification and quality features,” according to the US FDA.
Validation meaning in hindi
Answer: किसी उपकरण या प्रक्रिया की वैधता या सटीकता की जाँच या सिद्ध करने की क्रिया. डिजाइन प्रक्रिया के चरण से लेकर व्यावसायिक उत्पादन तक डेटा का संग्रह और मूल्यांकन।
जो वैज्ञानिक साक्ष्य के साथ लिखित प्रलेखित प्रमाणस्थापित करता है, कि यह प्रक्रिया एक गुणवत्ता वाले उत्पाद को मज़बूती से वितरित करने में सक्षम है। यह साबित करना कि एक प्रक्रिया या प्रणाली अपनी पूर्व निर्धारित आवश्यकताओं और गुणवत्ता मानकों को पूरा करती है।
What is Qualification?
Conducted to build confidence that auxiliary systems and process equipment can reliably operate within predetermined boundaries and tolerances.Offers written proof that the subject equipment has been installed in accordance with the specification (manufacturer’s advice) and will reliably achieve and maintain key process parameters. As part of a wider validation effort, a subset of validation is frequently performed.
Comparison between Validation and Qualifications
- Validation and Qualifications are essential components of the same concept.
- Qualification is normally used for equipment, utilities, and systems.
- Validation is normally used for processes and methods.
- In this sense, qualification is often a part (the initial stage) of validation but the individual qualification steps alone do not constitute process validation.
- Process validation cannot take place without first carrying out the qualification.
WHY VALIDATION?
- Assures Quality.
- Regulatory Requirement.
- Reduces Cost.
- It’s the LAW.
Validation Life Cycle Process
Validation should not be viewed as a one-off event. Validation must follow a structured documented process applying a lifecycle approach having the following key elements:
- Initial Validation
- Validation Review and
- Change Management
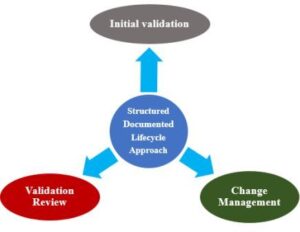
Validation Review Structured Documented Lifecycle Approach:
From the initial requirements definition to maintenance and eventually system decommissioning, validation covers the entire life of the system. Validation Documentation are:
1. Validation Master Plan (VMP)
2. Validation Protocols (VP)
3. Validation Reports (VR)
4. SOP Standard Oprating Procedures
Validation Master Plan:
Contains key elements of the validation program. Concise, clear, contains at least:
• A validation policy.
• The organizational structure of validation activities.
• Summary of facilities, systems, equipment, and processes validated (and to be validated).
• Documentation format (e.g. protocol and report).
• Planning and scheduling.
• Change control and references to the existing documents.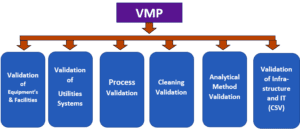
Validation Protocol in Validation and Qualifications.
• A validation protocol is detailed documentation relating to a specific part of the validation process.
• Outlines tests to be carried out, the acceptance criteria, and the information that must be recorded.
• Defines the approval process for the validation.
• Describes the procedure to be followed for performing validation.
• Objectives of the validation/qualification study, the site of the study, and the responsible personnel.
• Description of the equipment to be used (including calibration before and after validation)
• SOPs to be followed (e.g. the operation and cleaning of the equipment) and the standards and criteria for the relevant products and processes.
• The type of validation and time/frequency should also be stipulated.
• Processes and/or parameters to be validated (e.g. mixing times, drying temperatures, drying times, physical characteristics, content uniformity, etc.)
Validation Report in Validation and Qualifications.
- Record of results obtained during the performance of the validation.
- Reflects the final test results and other documents such as instrument calibration certificates.
- The basis on which the decision is taken on whether a particular process is judged to be validated.
- Includes evaluation, analysis, and comparison of results with acceptance criteria by the responsible personnel.
- Results should meet acceptance criteria and satisfy the stated objective.
- Refer to the protocol, state details of material, equipment, programs, and cycles used, together with details of procedures and test methods.
- Include recommendations on the limits and criteria to be applied to all future production batches.
- Protocol and the report may be combined into a single set of documents.
- The protocol is approved as a form on which the test results are recorded as they become available.
QUALIFICATION:
Types ( Stages) of Qualification in Validation and Qualifications :
- Design qualification (DQ)
- Installation qualification (IQ)Operational qualification (OQ)
- Performance qualification (PQ)
Commissioning:
- Include equipment start-up, adjustments, fine-tuning, cycle development, testing, and documentation flexibility.
- Leaves the equipment in a “State of Control”, which ensures the equipment is ready for formal qualification, testing, and approval.
- Supplements validity and regulatory compliance.
- Checks to verify the system or equipment is built to design specifications.
- No safety issues.
- Takes place at the manufacturer’s premise (FAT) or the User/Client’s Premise (SAT).
Design qualification (DQ):
- Process of completing and documenting design reviews to illustrate that all quality aspects have been fully considered at the design stage.
- Ensure that all the requirements for the final systems have been clearly defined at the start.
- In other words, has it been designed and selected correctly?
- Before purchasing, manufacturing equipment.
- Collection of data about similar equipment available in the market.
- Assessing your needs.
- Resources to buy, operate, space and maintenance they would need, etc.
- Making the decision.
Installation Qualification (IQ):
- Process of checking the installation, to ensure that the components meet the approved specification and are installed correctly, and to see how that information is recorded.
- Ensure that all aspects (static attributes) of the facility or equipment are installed correctly and comply with the original design.
- Considerations include:
- Equipment design features (i.e. materials of construction cleanability, etc.)
- Installation conditions (functionality, utilities, wiring, etc.)
- Calibration, preventative maintenance, cleaning schedules; safety features
- Supplier documentation, prints, drawings and manuals, software documentation
- Environmental conditions (such as clean room requirements, temperature, and humidity)
- Spare parts list E.g., a manufacturing equipment
- After purchasing (or critical repair).
- Place it in its intended place.
- Connect with other equipment, electric power, and material flow devices.
- Collect its documents including Operation Manual, etc.
- It is formally “released”: it is ready for work.
Operational Qualification
- Process of testing to ensure that the individual and combined systems function to meet agreed performance criteria and to check how the result of testing is recorded.
- To ensure that all the dynamic attributes comply with the original design.
- Does it work correctly?
- Assuring that the critical components and systems are capable of operating within established limits and tolerances
- “Worst case” (Proven Acceptable Range) demonstration that the equipment will perform as expected while operating at the extremes of the proposed range of operation.
Considerations include:
- Process control limits (e.g. time, temperature, pressure, line speed, setup conditions)
- Software parameters; starting material specifications
- Process operating procedures; material handling requirements
- Process change control; training; short-term stability and capability of the process, (latitude studies or control charts)
- Risk analysis and potential failure modes, action levels, and worst-case conditions (Failure Mode and Effects Analysis, Fault Tree Analysis E.g., a manufacturing equipment
- ”Model manufacturing” experiments with model materials, similar to those to be used in the real manufacture
- g. qualifying an autoclave, we use culture-media
- Permit the acceptable fluctuations of parameters, even set” worst conditions”
Performance Qualification
- Also called process qualification.
- Process of testing to ensure that the individual and combined systems function to meet agreed performance criteria on a consistent basis and to check how the result of testing is recorded.
- To ensure that the criteria specified can be achieved on a reliable basis over a period of time.
- Does it produce the product correctly?
In Validation and Qualifications, PQ includes:
- Actual product and process parameters and procedures established in OQ.
- Capabilities of process assurance.
- Acceptability of the medicine or products.
- Process stability over time and reproducibility.
- Similar to the Operational Qualification, but the real manufacturer is running, Permits accepted fluctuations up to their limits.
- All SOP”s, personnel, equipment, systems, Utilities, and materials are integrated. That verifies the pharmaceutical-grade utility, environment, equipment, or support system produces the required output.
- Production is done under conditions that simulate those planned to be used during the actual manufacturing.
- Change Control.
- Policy and procedure.
- Risk assessment.
- Authorization.
- Failure to properly document changes to the system means validation of the process.
Changes that Require Re-validation in Validation and Qualifications :
- Site Changes.
- Software changes, Controllers changes.
- Operational changes.
- Change of source of the material.
- Change in the process.
- Significant equipment change.
- Production area changes.
- Support system changes.
Validation Types in Validation and Qualifications:
- Process Validation
- Analytical Method Validation
- Cleaning Validation
- Water Systems Validation
- Computerized System Validation
Process Validation
- Means of ensuring, and providing documentary evidence that processes (within their specified design parameters) are capable of repeatedly and reliably producing a finished product of the required quality.
- PV should be completed prior to commercial manufacturing.
- Where this is not possible, it may be necessary to validate processes during routine production.
Prospective validation:
- Prospective validation is carried out during the development stage.
- Includes division of the production process into separate steps
- Analysis of potentially critical points in the manufacturing process e.g. mixing times, or temperature.
- Trials are carried out in which these steps and critical points are simulated and the effect on the process is assessed.
- Periodic Validation Review.
- Change Management Program.
- Periodic re-qualification.
- Validation Maintenance.
Cleaning Validation
Basic Considerations in Validation and Qualifications:
- Cleaning mechanisms: Mech. action, dissolution, chem. reaction, detergency.
- Cleaning Procedure: SOPs, tools & materials.
- Cleaning method: CIP, COP, manual cleaning.
- Detection of the impurities/contaminations.
- Selection of the Worst-case.
- Clean Equipment Hold times study.
- Best critical Sampling: Sampling Location, rinse period, swab, surface area, swab recovery, and analytical method.
Analytical Method Validation in Validation and Qualifications:
• Specificity
• Linearity
• Accuracy
• Precision
• Robustness
• LOD
• LOQ
Water Systems Validation and Qualifications are conducted in 3 phases:
- Phase 1: 2 to 4 weeks of intensive system monitoring, develop operating ranges, implement & refine SOPs.
- Phase 2: 2-4 weeks deploy refined SOPs, demonstrate operation within established ranges, and demonstrate the production of required quality & quantity.
- Phase 3: 1 year to verify long-term control, and demonstrate consistent production of required quality & quantity—ongoing system monitoring.
Computerized Systems Validation:
Basic Considerations are:
• System specification hardware, software, and user training.
• Functional specification testing, operating & maintaining the system.
• Security controls against unauthorized data manipulation, and audit trail.
• Backups secure data storage.
Conclusion:
- Validation and Qualifications is an essential component of GMP.
- Validation and Qualifications help to achieve the goal of assuring product quality, safety, and efficacy.
- Offers huge business benefits.
- The lifecycle approach covers entire systems and processes and must be structured, planned, and documented in Validation and Qualifications.
- Validation maintenance is an essential element of the validation lifecycle.
- Validation and Qualifications in the necessary part of parma industries.
Importance of validation in the pharmaceutical industry
Validation is a critical process in the pharmaceutical industry that ensures the safety, efficacy, and quality of pharmaceutical products. Here are some key reasons why validation is important in the pharmaceutical industry:
Compliance with Regulatory Requirements: Validation is a regulatory requirement for pharmaceutical manufacturing. Regulatory agencies such as the FDA, EMA, and WHO require pharmaceutical companies to validate their manufacturing processes to ensure that the products meet the required quality standards.
Risk Management: Validation helps to identify and mitigate risks associated with the manufacturing process. This includes identifying potential sources of contamination, ensuring that critical process parameters are controlled, and minimizing the risk of product variability.
Consistency and Quality: Validation ensures that manufacturing processes are consistent and reproducible, resulting in high-quality pharmaceutical products. This helps to ensure that patients receive medication that is safe and effective.
Cost-Effective Manufacturing: Validation helps to optimize manufacturing processes, reducing costs and improving efficiency. This includes identifying areas for improvement, reducing waste, and optimizing process parameters.
Continuous Improvement: Validation is an ongoing process that involves continuous monitoring and improvement of the manufacturing process. This helps to ensure that the manufacturing process remains in control and that the quality of the product is consistently maintained.
In summary, Validation and Qualifications are essential in the pharmaceutical industry to ensure compliance with regulatory requirements, manage risk, maintain consistency and quality, optimize manufacturing processes, and continuously improve the manufacturing process. Validation and Qualifications are a critical part of pharmaceutical manufacturing and help to ensure that patients receive safe and effective medication.
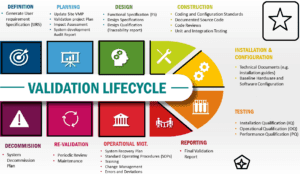
Importance of Validation and Qualifications in the pharmaceutical industry
Validation and Qualification are important in the pharmaceutical industry to ensure that products are safe, effective, and meet regulatory requirements. Here are some key reasons why Validation and Qualifications are important in the pharmaceutical industry:
Compliance with Regulatory Requirements: Validation and Qualifications are regulatory requirements for pharmaceutical manufacturing. Regulatory agencies such as the FDA, EMA, and WHO require pharmaceutical companies to validate and qualify their manufacturing processes to ensure that the products meet the required quality standards.
Risk Management: Validation and Qualifications help to identify and mitigate risks associated with the manufacturing process. This includes identifying potential sources of contamination, ensuring that critical process parameters are controlled, and minimizing the risk of product variability.
Consistency and Quality: Validation and Qualifications ensure that manufacturing processes are consistent and reproducible, resulting in high-quality pharmaceutical products. This helps to ensure that patients receive medication that is safe and effective.
Cost-Effective Manufacturing: Validation and Qualifications help to optimize manufacturing processes, reducing costs and improving efficiency. This includes identifying areas for improvement, reducing waste, and optimizing process parameters.
Continuous Improvement: Validation and Qualifications are ongoing processes that involve continuous monitoring and improvement of the manufacturing process. This helps to ensure that the manufacturing process remains in control and that the quality of the product is consistently maintained.
In summary, Validation and Qualifications are essential in the pharmaceutical industry to ensure compliance with regulatory requirements, manage risk, maintain consistency and quality, optimize manufacturing processes, and continuously improve the manufacturing process. It is a critical part of pharmaceutical manufacturing and helps to ensure that patients receive safe and effective medication.
Frequently Asked Questions (FAQ)
For more information about Validation and Qualifications and any draft documents, please write us at: admin@flairpharma.com
You may also click for guidelines here.

Quality Assurance Pharmaceutical and Biologics Guidelines