Data integrity in the best practices always allows organizations to fully understand their data. Data updates, additions, and deletions must be monitored and audited. The company must ensure that when data is moved from one system to another, or transformed, those transformations are the only thing that happens to the data. Procedures must be put in place so data remains accurate and complete as it flows through the organization. All pharma organizations have completion to follow the data integrity Policy. So the data integrity in pharma 4.0 and in CCS plays a key role.
The degree to which the data is complete, consistent, accurate, trustworthy, and reliable, and that these characteristics of data are maintained throughout the data lifecycle. The information should be gathered and kept in a secure way such that it is readable, authentic, and correct. It should also be attributable and recorded contemporaneously. Assuring data integrity requires appropriate quality unit and risk management systems, including adherence to sound scientific principles and good documentation practices.
Data integrity ‒ requirements for complete, consistent, and accurate data.
It is impossible to emphasize the significance of data integrity in preventing data loss or a data leak: in order to keep your complete data safe from outsiders from bad intentions, Also you have to make sure about your employees are treating data properly. You can make sure that sensitive data is never misclassified or wrongly stored, putting you at risk, by implementing the required data validation and error checking.
Types of data integrity in pharma
Understanding the two types of data integrity—logical and physical—is necessary for maintaining data integrity. Both are groups of procedures and techniques that guarantee data integrity in relational and hierarchical databases.
The physical Integrity
This involves preserving the accuracy and completeness of the data both during storage and retrieval. Physical integrity is in jeopardy when disasters occur, the power goes out, or database processes are interfered with by hackers. The inability of data processing managers, system programmers, applications programmers, and internal auditors to access accurate data can also be caused by human mistakes, storage deterioration, and a variety of other problems.
logical consistency
Data in a relational database is used in a variety of ways while remaining intact thanks to logical integrity. Physical integrity shields data against human error and hackers, while logical integrity accomplishes it in a very different way. This logic consistency/ integrity is further divided into 4 types:
- Entity Integrity
Relational databases that store data in tables that can be utilized and linked in a variety of ways have entity integrity as a feature. Identifying a piece of data depends on primary keys and uniquely identifying values being formed. This makes sure that fields in a table cannot be null and that data cannot be listed more than once. - The Reference Consistency
A collection of methods known as referential integrity ensures that data is preserved and used properly. Rules governing the use of foreign keys are ingrained in database structures, ensuring that only acceptable data deletions, modifications, and revisions can be made. This can assure data accuracy and prevent data duplication. - Domain Reliability
A set of procedures known as domain integrity ensures the accuracy of data points within a domain. A set of authorized values for a table’s columns, along with limitations and controls that establish restrictions on the volume, structure, and nature of the data that can be entered, serve to identify a domain. - Personal Integrity
User-defined integrity refers to the creation of data integrity rules and constraints by users in accordance with their unique needs. This is typically used when other integrity procedures won’t protect an organization’s data, enabling the development of rules that take data integrity safeguards into account.
The brief concept of data integrity as per the cGMP.
As per cGMP & Good Clinical Practice by the International Council For Harmonisation (ICH E6). Data accuracy, uniformity, and totality are all closely related to data integrity. Data should be complete, uniform, transparent, chronologically documented, original or a true copy, and precise (ALCOA) (USFDA).
ALCOA Rather than ‘ALCOA’. ALCOA is attributable, legible, contemporaneous, original, and accurate and the ‘+’ refers to complete, consistent, enduring, and available. Data governance practises should guarantee that data is comprehensive, steadily sustained, and accessible as guided by (MHRA).
How well data are complete, consistent, accurate, trustworthy, and reliable, and how well they keep these qualities over the course of their existence.
TYPES OF DATA
DATA: Facts, Figures, and statistics collected together for Reference Analysis.
The Raw Data: The original Record (data), also known as the initial information capture, is referred to as raw data. whether recorded on paper or electrical Information that is originally captured in a dynamic state should remain available in that state.
The Source Data: Incorporation of all the data from both the original records and the certified copies of the original documents that were used to reassemble and assess the inquiry.
Electronic Record: FDA Regulation defined as any combination of text, graphics, data, audio, pictorial or other information Represented in digital form that is created, modified maintained, archived, retrieved, or distributed by
Meta Data: Data that explain the structure, data elements, interrelationships, and other features of data, such as audit trails, are known as metadata. Moreover, metadata enables data to be linked to a specific person ( or if automatically generated, to the original data source). An essential component of the original record is the metadata.
Audit Trail: Audit trails are metadata that are a record of GMP/GDP Critical information about the creation, modification, or deletion of relevant data, which permits the reconstruction of GMP/GDP activities. Audit trails include who, what, when, and why chronologically action is performed.
Data governance:
Data governance is the collection of policies and procedures that guarantee the accuracy of the data. These arrangements make sure that data is recorded regardless of the process, format, or technology used to create it., processed, retained, retrieved, and used will ensure a complete, consistent, and accurate record throughout the data life cycle.
Data/Information Lifecycle:
The process through which data is created, used for decision-making, processed, reported, checked, saved, and then eventually deleted at the end of the retention period is known as the data lifecycle.
You may also read about the Data integrity in Contamination Control strategy (CCS)
Data Interpretation And Good documented practices
- Information is considered as data Original Records.
- The actual copies of original records.
- Sourced data ( printout from HMI e.g. STP, CIP, autoclave balance, HPLC, FTIR).
- Metadata, Reports printouts.
- Any information Recorded at the time of activity performed
Data allows to reconstruct and evaluate carried out GxP activity, GDP are those measures that collectively and individually ensure documentation, whether paper or electronic are following the principles of ALCOA+
Documents used for GMP purposes should comply with Gdocp i.e BMR, logbook, specification, SOP, analytical method, protocol, qualification Doc, COFA, TA, PQRDocument handling procedure i.e retention, Archiving, periodic Review.
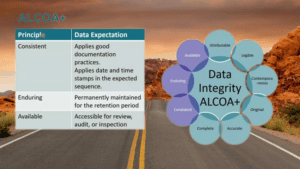
| ALCOA | ALCOA+ |
| Attributable | Consistent |
| Legible | Complete |
| Contemporaneous | Enduring |
| Original | Available |
| Accurate |
ALCOA
- Attributable: Clear who has captured and documented the data.
- Legible: Readable and stay in permanent format throughout the lifecycle.
- Contemporaneous: Recorded at the time of activity.
- Original: First recorded data, not a true copy.
- Accurate: True representation of fact.
ALCOA+
- Consistent: Recorded in some way.
- Complete: Complete pack of data Including metadata.
- Enduring: Long lasting durable Storage (Paper, electronic)
- Available: Retrievable and accessible for review
The Common Issues of Data Integrity
- International data falsification or manipulation
- Poor documentation practices impact the reliability of the data.
- Lack of control related to software, computerized systems, or instruments
- Lack of Review process to ensure detection ability of any data integrity gaps
- Shared identify/ passwords
Data Integrity Failure Examples
- Lack of controlled access to computer systems.
- Trial” HPLC injections outside a quality structure.
- Deleted data.
- Not recording activities contemporaneously.
- Fabricating data.
- Copying existing data as new data.
- Discarding or deleting results with no justification and re-running/retesting samples to present better results.
How to Minimize the Risk of Data Integrity
- Follow good documentation practice.
- Follow ALCOA+.
- Audit trail.
- Quality Management System.
- Internal audit/self-inspection.
- Personal training.
- Computer system validation.
- Data backup and recovery.
CONCLUSION
In the pharmaceutical industry data integrity play an important role to maintain the quality of the product as well as patient safety. A process of maintenance and assurance of accuracy and consistency of the data over its entire life cycle. The integrity and trustworthiness of the pharmaceutical product. Data integrity helps in building trust between regulatory agencies and the industry as a whole.
You may read the best book for Data Integrity and Data Governance: Practical Implementation in Regulated Laboratories.
