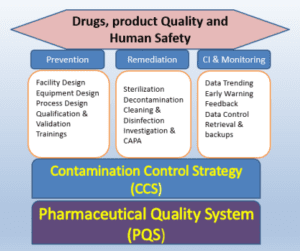
The HVAC qualification process in pharmaceutical plants holds immense importance in ensuring the integrity, safety, and efficacy of drug manufacturing processes. Here are the key reasons why HVAC qualification is crucial in pharmaceutical plants:
- Product Quality Assurance: HVAC systems create and maintain critical environmental conditions required for drug manufacturing, including temperature, humidity, and airflow. Proper qualification ensures that these conditions remain consistent, safeguarding the quality and stability of pharmaceutical products.
- Compliance with Regulatory Standards: The pharmaceutical industry is highly regulated, and adherence to Good Manufacturing Practices (GMP) is paramount. HVAC qualification demonstrates compliance with regulatory guidelines, providing assurance to authorities that the manufacturing environment meets the required standards.
- Contamination Control: HVAC systems play a vital role in controlling contamination within cleanrooms and controlled areas. Qualification verifies the effectiveness of air filtration and containment, minimizing the risk of product contamination during manufacturing.
- Patient Safety: Pharmaceutical products directly impact patient health. Qualified HVAC systems prevent cross-contamination and ensure the production of safe and effective medications, protecting patient well-being.
- Process Consistency: Variation in environmental conditions can lead to batch-to-batch inconsistencies in pharmaceutical production. HVAC qualification ensures that processes remain consistent, enhancing product uniformity and reliability.
- Data Integrity: Validation processes involve rigorous data collection and analysis. This emphasis on data integrity provides a robust foundation for decision-making, process improvements, and troubleshooting.
- Risk Management: HVAC qualification includes risk assessments to identify potential vulnerabilities and mitigations. This proactive approach helps prevent environmental deviations that could jeopardize product quality and patient safety.
- Energy Efficiency: An adequately qualified HVAC system operates efficiently, reducing energy consumption and lowering production costs, making pharmaceutical manufacturing more sustainable.
- Operational Efficiency: A validated HVAC system operates reliably and optimally, minimizing downtime and maintenance disruptions, thus improving overall manufacturing efficiency.
- Cross-Functional Collaboration: HVAC qualification encourages collaboration among various departments, such as engineering, quality assurance, and production, fostering a holistic approach to facility management.
- Validation Master Plan (VMP): Creating a VMP as part of the qualification process establishes a systematic strategy for validation activities, facilitating long-term planning and resource allocation.
- Continuous Improvement: Regular requalification and periodic reviews promote a culture of continuous improvement, ensuring that HVAC systems adapt to changes in the pharmaceutical environment and technology.
In conclusion, HVAC qualification is the backbone of pharmaceutical manufacturing. It guarantees that critical environmental conditions are maintained, compliance with regulatory standards is upheld, and patient safety remains uncompromised. With its multifaceted impact on product quality, contamination control, and operational efficiency, HVAC qualification plays a pivotal role in the pharmaceutical industry’s
Important Aspects of the HVAC Qualification in the pharmaceutical plants
- Design Specification: Establishing stringent design specifications to ensure the HVAC system aligns with the plant’s unique needs.
- Installation Qualification (IQ): Comprehensive examination to verify correct installation and interconnection of HVAC components.
- Operational Qualification (OQ): Performance testing under normal operating conditions to demonstrate system functionality.
- Performance Qualification (PQ): Meticulous validation of HVAC’s consistent ability to maintain precise environmental conditions.
- Regulatory Compliance: Adherence to GMP and other regulatory requirements throughout the qualification process.
- Temperature Mapping: Ensuring uniform temperature distribution across critical areas.
- Airflow Visualization Studies: Verifying proper air circulation and containment within cleanrooms.
- Filter Integrity Testing: Ensuring efficient filtration to maintain air quality.
- Particle Count Testing: Measuring airborne particle levels to guarantee a controlled environment.
- Humidity Control: Validating the HVAC’s capacity to maintain specific humidity levels.
- Recovery Testing: Assessing the system’s ability to recover environmental conditions after disturbances.
- Documentation and Record-keeping: Thorough documentation of all validation activities and results.
- SOPs and Protocols: Developing comprehensive Standard Operating Procedures and validation protocols.
- Training and Competency: Ensuring personnel involved in HVAC qualification are trained and competent.
- Change Control and Risk Assessment: Managing changes to the HVAC system and assessing associated risks.
- Periodic Requalification: Scheduling regular requalification to maintain continued compliance.
- Validation Report: Compiling a comprehensive validation report summarizing all findings.
- Validation Master Plan (VMP): Developing a VMP outlining the validation strategy and scope.
- Validation Team: Assembling a multidisciplinary team with expertise in HVAC systems and pharmaceutical manufacturing.
- Equipment Calibration: Ensuring all measuring instruments are calibrated and traceable to standards.
- Validation Testing in Empty and Loaded Conditions: Evaluating HVAC performance under various load conditions.
- Data Integrity: Ensuring accurate and reliable data collection and recording throughout validation.
- Quality Risk Management: Implementing risk-based approaches to prioritize validation efforts.
- Validation Deviations and CAPA: Addressing and documenting deviations and implementing corrective and preventive actions.
- Periodic Review: Conducting periodic reviews to assess the continued suitability of HVAC qualification.
- Critical Utilities Validation: Validating other critical utilities that may affect HVAC system performance.
- Interlocking Systems: Verifying interlocking with critical processes to prevent non-compliant operations.
- Third-party Audits: Facilitating third-party audits for an objective assessment of HVAC qualification.
Remember that this list serves as a starting point, and the specific aspects of HVAC qualification may vary depending on the pharmaceutical plant’s unique requirements and regulatory guidelines.
HVAC Qualification as per the ISO 14644 : 2019 in the pharmaceutical Plants
| Aspect of HVAC Qualification | Description |
|---|---|
| Classification of Cleanrooms and Controlled Environments | Defining different classes of air cleanliness based on allowable concentrations of airborne particles. |
| Performance Testing and Validation | Conducting rigorous performance testing to verify that HVAC systems meet cleanliness levels for designated classes. |
| Particle Counting | Performing measurements to assess airborne particulate cleanliness levels within cleanrooms and controlled areas. |
| Filter Integrity Testing | Assessing the efficiency and integrity of HVAC system filters to ensure effective capture and retention of particles. |
| Installation and Operational Qualification (IQ/OQ) | Verifying proper installation and functionality of HVAC systems through IQ and OQ protocols. |
| Performance Qualification (PQ) | Comprehensive testing of HVAC system performance under dynamic operating conditions to maintain required parameters. |
| Monitoring and Contamination Control | Continuous monitoring of cleanrooms and controlled environments, including HVAC systems, to control particle levels. |
| Personnel Training and Competency | Providing personnel with adequate training to ensure proper handling and understanding of ISO 14644:2019 requirements. |
| Documentation and Record-Keeping | Thoroughly documenting HVAC qualification processes, test results, protocols, and reports for regulatory compliance. |
Steps of the HVAC Qualification:
The HVAC qualification process in pharmaceutical plants involves several key steps to ensure the system’s compliance, functionality, and capability to maintain precise environmental conditions. Here are the steps involved in HVAC qualification:
- User Requirement Specification (URS):
- Defining the requirements and expectations for the HVAC system based on the pharmaceutical plant’s needs, regulatory guidelines, and industry standards.
- Design Qualification (DQ):
- Reviewing and approving the HVAC system’s design, including equipment specifications, layout, and documentation.
- Installation Qualification (IQ):
- Verifying that the HVAC system is installed correctly according to approved design documents and manufacturer’s recommendations.
- Operational Qualification (OQ):
- Conducting performance testing under normal operating conditions to ensure the HVAC system functions as intended.
- Performance Qualification (PQ):
- Validating the HVAC system’s ability to consistently maintain specific environmental conditions, such as temperature, humidity, and airflow, over an extended period.
- Temperature Mapping and Distribution Studies:
- Performing temperature mapping studies to ensure uniform temperature distribution within critical areas of the pharmaceutical plant.
- Airflow Visualization Studies:
- Conducting airflow studies to verify proper air circulation and containment within cleanrooms and controlled areas.
- Filter Integrity Testing:
- Testing the efficiency of filters to ensure effective air filtration and contaminant removal.
- Particle Count Non Viable Testing:
- Measuring airborne particle levels to assess the cleanroom’s cleanliness and compliance with regulatory standards.
- Viable Monitoring:
- The process of assessing the presence and concentration of living microorganisms, such as bacteria and fungi, in a given environment. This type of monitoring is essential in various industries, including pharmaceuticals, biotechnology, healthcare, and food production, where the control of microorganisms is critical to ensure product quality, safety, and compliance with regulatory standards.
- Humidity Control Testing:
- Validating the HVAC system’s ability to maintain specific humidity levels within defined tolerances.
- Recovery Testing:
- Assessing the system’s ability to recover environmental conditions after disturbances, such as door openings or power failures.
- Change Control and Risk Assessment:
- Managing changes to the HVAC system and conducting risk assessments to evaluate potential impacts on validation.
- Validation Report:
- Compiling all validation activities, test results, and observations into a comprehensive validation report.
- Validation Master Plan (VMP):
- Creating a detailed plan outlining the validation strategy, scope, responsibilities, and timeline.
- Training and Competency:
- Providing training to personnel involved in HVAC qualification to ensure they understand the procedures and requirements.
- Validation Deviations and Corrective Actions:
- Addressing and documenting any deviations from acceptance criteria and implementing corrective and preventive actions as necessary.
- Periodic Requalification:
- Scheduling regular requalification to ensure ongoing compliance and system performance.
- Validation Closure:
- Officially closing the validation process after successful completion of all qualification steps and documentation.
Throughout the HVAC qualification process, clear documentation, adherence to regulatory guidelines, and collaboration among cross-functional teams play pivotal roles in ensuring a successful and compliant outcome.
Advantages of the HVAC Qualification in the pharmaceutical Plants
| Advantages of HVAC Qualification in Pharmaceutical Plants |
|---|
| 1. Product Quality Assurance. |
| 2. Compliance with Regulatory Standards. |
| 3. Contamination Control. |
| 4. Patient Safety. |
| 5. Process Consistency. |
| 6. Data Integrity. |
| 7. Risk Management. |
| 8. Energy Efficiency. |
| 9. Operational Efficiency. |
| 10. Cross-Functional Collaboration. |
| 11. Validation Master Plan (VMP). |
| 12. Continuous Improvement. |
Frequently Asked Questions:
What is HVAC qualification in pharmaceuticals?
Answer: HVAC qualification in pharmaceuticals refers to the process of validating and verifying Heating, Ventilation, and Air Conditioning systems to ensure they meet specific regulatory and quality standards for maintaining clean and controlled environments in pharmaceutical manufacturing facilities.
Why is HVAC qualification essential in pharmaceuticals?
Answer: HVAC qualification is crucial in pharmaceuticals to ensure the controlled environment’s cleanliness, maintain product quality, and comply with regulatory requirements.
What are the key steps involved in HVAC qualification?
Answer: The key steps in HVAC qualification include Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
What is the purpose of Installation Qualification (IQ)?
Answer: IQ verifies that the HVAC system is correctly installed, meets design specifications, and complies with manufacturer’s recommendations.
What is the purpose of Operational Qualification (OQ)?
Answer: OQ tests the HVAC system under normal operating conditions to ensure it functions as intended and meets performance requirements.
What is the purpose of Performance Qualification (PQ)?
Answer: PQ assesses the HVAC system’s ability to consistently maintain specific environmental conditions over an extended period.
How is particle count testing performed in HVAC qualification?
Answer: Particle count testing involves measuring airborne particulate levels in cleanrooms to assess the effectiveness of the HVAC system’s filtration.
What is the significance of filter integrity testing in HVAC qualification?
Answer: Filter integrity testing evaluates the efficiency and integrity of HVAC system filters, ensuring they effectively capture and retain particles.
Why is personnel training and competency important in HVAC qualification?
Answer: Personnel involved in HVAC qualification should receive proper training to handle the system and understand the qualification procedures.
What role does documentation play in HVAC qualification?
Answer: Comprehensive documentation is crucial to demonstrate compliance with regulatory standards and to maintain a record of the qualification process.
How does HVAC qualification help in contamination control?
Answer: HVAC qualification ensures that the system effectively controls airborne particulate contamination, minimizing the risk of product contamination.
What is the significance of risk assessment in HVAC qualification?
Answer: Risk assessment identifies critical areas and potential contamination sources, helping to focus monitoring efforts on high-risk areas.
How does HVAC qualification support aseptic process validation in pharmaceuticals?
Answer: HVAC qualification is an integral part of aseptic process validation, ensuring the production of sterile drugs and biologics is free from contamination.
What are the key regulatory guidelines governing HVAC qualification in pharmaceuticals?
Answer: Regulatory guidelines such as ISO 14644:2019 and GMP outline requirements for HVAC qualification in pharmaceutical manufacturing.
Why is continuous monitoring important in HVAC qualification?
Answer: Continuous monitoring of cleanrooms and controlled environments, including HVAC systems, ensures ongoing compliance and the prevention of deviations in environmental conditions.

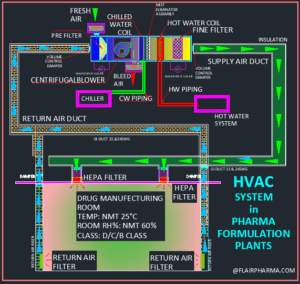

In a Pharmaceutical Formulations Facility we have an Area we’re the Airflow and Exhaust are on the Ceiling side. The Laminarity is well achieved. But many Auditors have pinpointed that a section in a room is dead as the Air is not reaching there. How to tackle this issue.
To cater this dead area provide then the temperature & RH Mapping of these area. Also find out & indicates the HOT point of the rooms.
Kindly share with reference frequency of PAO Test & Air Balancing in OSD & Sterile Area.