A pH meter is an essential tool in the pharmaceutical industry for measuring the acidity or alkalinity of solutions used in the production of drugs, as well as monitoring the pH of drug products. Accurate pH measurements are critical in ensuring product quality and safety, as pH can affect drug stability, solubility, and efficacy. pH meters used in the pharmaceutical industry must meet strict regulatory requirements and be calibrated regularly to maintain accuracy. Advanced features such as automatic temperature compensation and data logging capabilities make pH meters a valuable asset in pharmaceutical research, development, and quality control processes.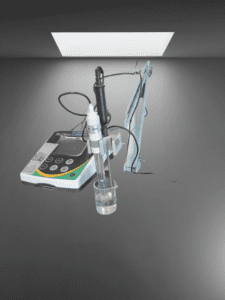
ph Meter
- Ensure the quality and safety of your pharmaceutical products with accurate pH measurements using our pH meters.
- Trust our pH meters to provide precise and reliable pH readings for your pharmaceutical processes and products.
- Achieve optimal pH control in your pharmaceutical operations with our range of high-quality pH meters and accessories.
- From research and development to production and quality control, our pH meters are essential tools for the pharmaceutical industry.

What is a pH Meter?
The probe contains a glass electrode that responds to changes in hydrogen ion concentration by generating a voltage signal. The reference electrode, usually a silver/silver chloride electrode, provides a stable reference potential for the measurement. The meter measures the voltage the probe generates and converts it to a pH reading.
pH meters are commonly used in many scientific fields, including chemistry, biology, and environmental science, to measure the pH of solutions such as water, soil, and biological fluids. In industry, pH meters are widely used in food and beverage production, pharmaceuticals, and water treatment to ensure product quality and safety. Advanced pH meters may also have features such as automatic temperature compensation, data logging capabilities, and the ability to measure other parameters such as conductivity and dissolved oxygen.
Relationship between pH values and hydrogen ion concentrations:
| pH Value | Hydrogen Ion Concentration |
| 0 | 1.0 x 10^-0 mol/L |
| 1 | 1.0 x 10^-1 mol/L |
| 2 | 1.0 x 10^-2 mol/L |
| 3 | 1.0 x 10^-3 mol/L |
| 4 | 1.0 x 10^-4 mol/L |
| 5 | 1.0 x 10^-5 mol/L |
| 6 | 1.0 x 10^-6 mol/L |
| 7 | 1.0 x 10^-7 mol/L (neutral) |
| 8 | 1.0 x 10^-8 mol/L |
| 9 | 1.0 x 10^-9 mol/L |
| 10 | 1.0 x 10^-10 mol/L |
| 11 | 1.0 x 10^-11 mol/L |
| 12 | 1.0 x 10^-12 mol/L |
| 13 | 1.0 x 10^-13 mol/L |
| 14 | 1.0 x 10^-14 mol/L |
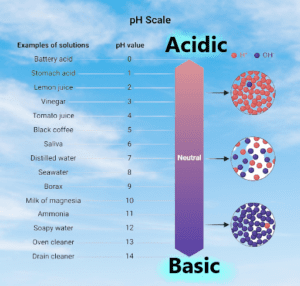
the pH of a solution decreases, the hydrogen ion concentration increases, making the solution more acidic. Conversely, as the pH of a solution increases, the hydrogen ion concentration decreases, making the solution more alkaline. The pH scale is logarithmic, which means that a change of one pH unit represents a tenfold change in hydrogen ion concentration.
Major technical parts of the ph Meter:
A pH meter is an instrument that measures the acidity or basicity of a solution. The major technical parts of a pH meter include:
- Electrode: The electrode is the most important part of a pH meter. It consists of a glass membrane and a reference electrode. The glass membrane is sensitive to hydrogen ions, and the reference electrode maintains a constant voltage.
- Calibration controls: A pH meter needs to be calibrated before use to ensure accurate readings. The calibration controls allow the user to adjust the pH meter to read accurately at known pH values.
- Display: The display is where the pH reading is shown. It can be a digital display or an analog meter.
- Temperature sensor: pH measurements are affected by temperature, so a temperature sensor is included in most pH meters. The temperature sensor is used to compensate for temperature changes and ensure accurate readings.
- Battery: The pH meter requires a battery to power the electronics.
- Amplifier: The amplifier amplifies the signal from the electrode to a level that can be read by the display.
- Circuit board: The circuit board contains the electronic components that control the pH meter.
- Housing: The housing protects the electronics and provides a handle for the user to hold the pH meter.
Applications of the ph meter in the pharmaceutical industry:
pH meters are widely used in the pharmaceutical industry for various applications such as:
- Quality control of raw materials: pH meters are used to measure the pH of raw materials such as active pharmaceutical ingredients (APIs), excipients, and solvents to ensure that they meet the required specifications.
- Formulation development: pH measurements are essential in the formulation development of drugs. pH meters are used to determine the optimal pH range for the drug product and to monitor the pH during the formulation process.
- Stability testing: pH is a critical factor that affects the stability of drug products. pH meters are used to monitor the pH of drug products during stability testing to ensure that they remain within the specified range.
- In-process control: pH meters are used for in-process control to ensure that the pH of the drug product is within the specified range during the manufacturing process.
- Cleaning validation: pH meters are used to measure the pH of cleaning solutions used in the cleaning validation of manufacturing equipment to ensure that they are effective in removing residues and contaminants.
- Dissolution testing: pH meters are used to measure the pH of dissolution media used in the dissolution testing of drug products. pH is an important factor that affects the solubility and dissolution of drugs.
- Microbiology: pH meters are used to measure the pH of growth media for microbiological testing. The pH of the media can affect the growth and survival of microorganisms.
Overall, pH meters are essential tools in the pharmaceutical industry for ensuring product quality, optimizing formulations, and monitoring critical processes.
The advantages and disadvantages of the pH meter.
| Advantages | Disadvantages |
| Highly accurate and precise measurements. | Requires calibration and maintenance. |
| Easy to use and portable. | The electrode may become contaminated or damaged. |
| Provides real-time measurements. | Temperature changes can affect the accuracy. |
| Can measure a wide range of pH values. | Requires a sample of the solution to be tested. |
| It can be used in a variety of industries and applications. | High initial cost. |
| It can be used to monitor pH changes over time. | It may require specialized training. |
| Provides quick results. | Limited to measuring pH only. |
 FAQ:
FAQ:
How accurate are pH meters?
Answer: The accuracy of a pH meter depends on various factors such as the quality of the electrode, the calibration of the meter, and the stability of the voltage reference. High-quality pH meters can provide accurate readings within ±0.01 pH units.
How often should I calibrate my pH meter?
Answer: It is recommended to calibrate your pH meter before each use to ensure accurate readings. If you use your pH meter frequently, it is recommended to calibrate it at least once a week.
How do I clean my pH meter?
Answer: You can clean your pH meter by rinsing the electrode with distilled water and wiping it with a soft cloth. Do not use soap or detergents as they can leave a residue that affects readings. You can also use a special cleaning solution designed for pH meters.
Can I use my pH meter to measure the pH of solid samples?
Answer: No, pH meters are designed to measure the pH of liquid solutions. For solid samples, you can make a slurry by mixing the sample with water and then measuring the pH of the resulting solution.
How do I store my pH meter?
Answer: It is recommended to store your pH meter in a dry and cool place with the electrode kept moist in a storage solution. Avoid storing the electrode in distilled water, as it can damage the electrode.
How do I know if my pH meter needs a new electrode?
Answer: If the readings of your pH meter become unstable or inaccurate, it may be time to replace the electrode. Additionally, if the electrode appears damaged or cracked, it should be replaced immediately.
What is the optimal temperature range for pH measurements?
Answer: The optimal temperature range for pH measurements is usually between 5-40°C. It is important to note that pH measurements are affected by temperature, so it is recommended to use a temperature sensor to compensate for temperature changes.
How do I troubleshoot my pH meter if it is not working properly?
Answer: If your pH meter is not working properly, the first step is to check the battery level and ensure that the electrode is properly connected. If the problem persists, try recalibrating the meter or replacing the electrode.
Can pH meters measure the pH of non-aqueous solutions?
Answer: Some pH meters are designed to measure the pH of non-aqueous solutions such as oils and creams. However, these types of solutions may require specialized electrodes and calibration procedures.
How do I choose the right pH meter for my needs?
Answer: When choosing a pH meter, consider factors such as the accuracy and precision required, the types of solutions you will be measuring, and the budget. High-end pH meters offer greater accuracy and precision, but also come with a higher price tag.
Do you know how water quality is Monitored in Pharmaceuticals?

 FAQ:
FAQ: