The Master Formula Record (MFR) is the backbone of pharmaceutical manufacturing. It dictates “what to make, how to make it, and what standards to meet.” Crafting an MFR is not merely a documentation exercise; it’s an orchestration of science, compliance, and process integrity. Below is a uniquely structured representation of how an MFR is prepared using a 4-Layer Matrix Model: Regulatory, Technical, Structural, and Verification.
Definition :
Master Formula Record (MFR) is a controlled, pre-approved document that provides comprehensive written instructions for the manufacturing process of a specific pharmaceutical product, ensuring consistency, quality, and compliance with regulatory standards.
Master Formula Record (MFR) prepration flow chart:

Types of MFR – Presented as a Table of Classification

Role-Based Responsibilities in MFR Management
| Department | MFR Role |
|---|---|
| R&D / Formulation | Draft initial version with scientific details |
| Production | Review for practicality, equipment fit, and process flow |
| Quality Assurance | Ensure GMP compliance, approve final version |
| Document Control | Assign version and archive history |
| Regulatory Affairs | Verify if MFR aligns with product dossier/CTD |
“Why is MFR Needed?”
| Element | Description | Unique Insight |
|---|---|---|
| GMP Requirement | As per Schedule M, EU GMP, WHO-GMP, and US FDA. | Ensures product consistency and patient safety. |
| Audit Backbone | Acts as a base document for internal, regulatory, or third-party audits. | Viewed as the “constitutional document” of a batch. |
| Validation Anchor | Used for process validation and lifecycle approach. | Establishes product lifecycle from paper to pill. |
| Data Integrity | Prevents undocumented changes, promotes traceability. | MFR is ALCOA+ compliant (Attributable, Legible, etc.) |
Verification and Uniqueness Checks
| Checkpoint | Methodology | Unique Value Added |
|---|---|---|
| Version Control | Document control number, revision log | Avoids outdated or unauthorized usage |
| Cross-functional Review | Inputs from QA, QC, RA, and Engineering | Builds multi-disciplinary robustness |
| Mock Run Testing | Pilot-scale or simulation batches | Validates MFR before actual use |
| Reconciliation Logic | Input/output balance with yield tracking | Ensures zero loss and traceability |
| Barcode/QR Integration | Optional for electronic MFRs | Enables quick traceability and automation |
Frequently asked questions (FAQ):
What is a master formulation record?
A Master Formulation Record (MFR) is a comprehensive recipe and procedural guide for manufacturing a specific pharmaceutical product, detailing ingredients, quantities, processes, and critical parameters.
What is the master formula guideline?
This refers to regulatory or organizational protocols that govern how an MFR must be structured, documented, reviewed, and approved, ensuring consistency and compliance in drug production.
What are the major components of master formula record?
| Component | Description |
|---|---|
| Product Details | Name, strength, dosage form |
| Batch Size | Target quantity or volume |
| Ingredients List | APIs and excipients with exact amounts |
| Manufacturing Steps | Sequential instructions |
| In-Process Controls | Parameters monitored during production |
| Packaging Instructions | Container-closure and labeling |
| Precautions | Warnings and safety measures |
| Equipment List | Machines/tools required |
What is an MFR document?
It’s a controlled, authorized document that serves as the definitive source of manufacturing instructions for a batch, designed to ensure batch-to-batch uniformity.
What is the purpose of MFR?
To ensure reproducibility, regulatory compliance, and product quality by providing standardized instructions for manufacturing.
What is MFR standard?
MFR standards are predefined norms (often based on GMP or FDA guidelines) that dictate the format, content, and approval process of the formulation record.
What is MFR and MVR?
- MFR = Master Formulation Record (planned procedure).
- MVR = Master Validation Record (strategy for validating processes or systems).
What is the MFR used for?
It’s used as the master blueprint for preparing every batch of a product, guiding operators and QA personnel during manufacturing.
What is the difference between MFR and CFR?
- MFR: A document defining product manufacturing.
- CFR (Code of Federal Regulations): Legal framework issued by the US government, containing GMP rules (e.g., 21 CFR Part 211).
What is the difference between MFR and BMR?
| Aspect | MFR (Master) | BMR (Batch) |
|---|---|---|
| Purpose | Standardized instruction set | Actual batch documentation |
| Usage | Created once | Created per batch |
| Type | Template | Execution record |
Who GMP documentation?
The World Health Organization (WHO) defines GMP documentation as all written records, instructions, and SOPs that ensure quality and traceability in pharmaceutical manufacturing.
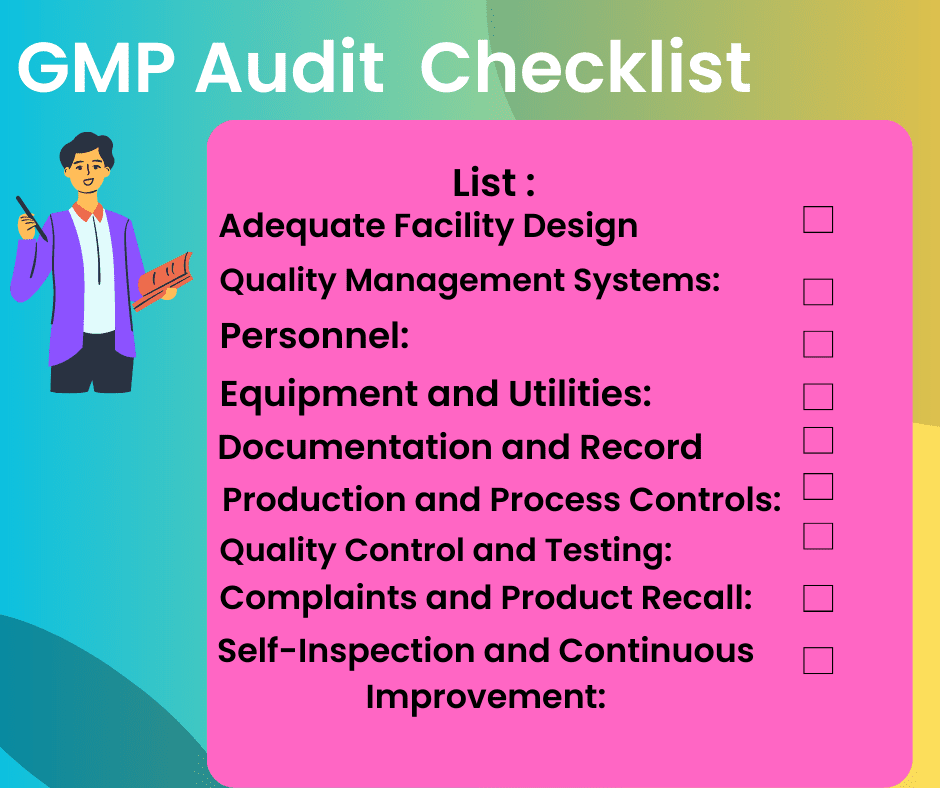
What is a master formulation record MFR?
It’s the authoritative protocol that provides detailed instructions for producing a pharmaceutical product in a consistent manner.
What is the full form of DMF?
DMF = Drug Master File — a submission to regulatory authorities containing detailed information about drug substances or excipients.
What is master record types?
Master records include:
- MFR (Master Formulation Record)
- Master Packaging Record
- Master Validation Protocol
- Master Equipment List
How is a master record made?
By compiling validated formulation steps, equipment requirements, QC parameters, and safety guidelines, then reviewing and approving it through QA/QC and regulatory bodies.
What is the master formula?
It is the complete, validated set of instructions used to consistently manufacture a pharmaceutical product.
What are the benefits of master formula?
- Batch uniformity
- Regulatory compliance
- Quality assurance
- Traceability
- Efficient training tool
What are the details of MFR?
It includes: formulation name, code, strength, ingredients, processing steps, yield, precautions, QC tests, and equipment.
What is the master schedule in MRP?
In Material Requirements Planning (MRP), the master schedule outlines forecasted production timelines and quantities to align supply chain with demand.
What is MFR on prescription?
In prescription contexts, MFR may refer to the manufacturer’s name rather than Master Formulation Record.
What is master record?
A high-level, controlled document that defines the standard procedure or composition of a product or process.
What does an MFR stand for?
MFR = Master Formulation Record.
What is the full form of CFR and MFR?
- CFR = Code of Federal Regulations
- MFR = Master Formulation Record
What is SOP in pharmaceutical industry?
SOP = Standard Operating Procedure — a step-by-step directive to perform routine operations consistently and in compliance with regulations.
How is MFR calculated?
In plastics/polymer science, MFR (Melt Flow Rate) = mass of polymer extruded in 10 minutes (g/10 min). Not to be confused with Master Formulation Record.
What is MFR format?
A typical MFR format includes:
- Header (product info)
- Purpose & Scope
- Ingredients list
- Equipment & tools
- Step-by-step instructions
- In-process controls
- Signature fields
How to convert MVR to MFR?
You don’t directly convert MVR to MFR. However, insights from a validated process (MVR) can be incorporated into MFR to update manufacturing instructions.
What is the difference between MFR and MVR?
| Feature | MFR | MVR |
|---|---|---|
| Focus | Product manufacturing | Process validation |
| Output | Batch preparation guidance | Validation data & conclusions |
| Usage | Production environment | QA/Validation departments |
What is MFR model code?
In polymer testing, MFR model codes identify specific melt flow indexer instruments. In pharma, “model code” is not typically applied to MFR.
What is a compounding SOP?
An SOP detailing how to mix or combine raw materials to create a final compounded preparation, ensuring accuracy, safety, and compliance.
What is master list of records?
A centralized, controlled document that lists all approved SOPs, forms, logs, and other GMP-related documents for tracking and auditing.
What is the difference between MFR and MFG?
- MFR: Master Formulation Record (document)
- MFG: Manufacturing (process or department)
How to prepare MFR?
Steps:
- Identify formulation and batch size
- Detail ingredients and specifications
- Write manufacturing steps clearly
- Include IPCs and packaging instructions
- Review for accuracy
- Approve by QA and Regulatory Affairs
- Version control and document release
