The technology of WFI Generation and Distribution Plant:
Water For injection is used as an excipient in the pharmaceutical industries. WFI will chemically and microbiologically purify water, and is produced by the change in phase and entrain separation. For WFI the purified water is evaporated with the help of raw steam or heaters and produces pure steam. Then this pure steam is condensed with the help of heat exchangers/ cooling water and found the condensed water which we called water for the injection.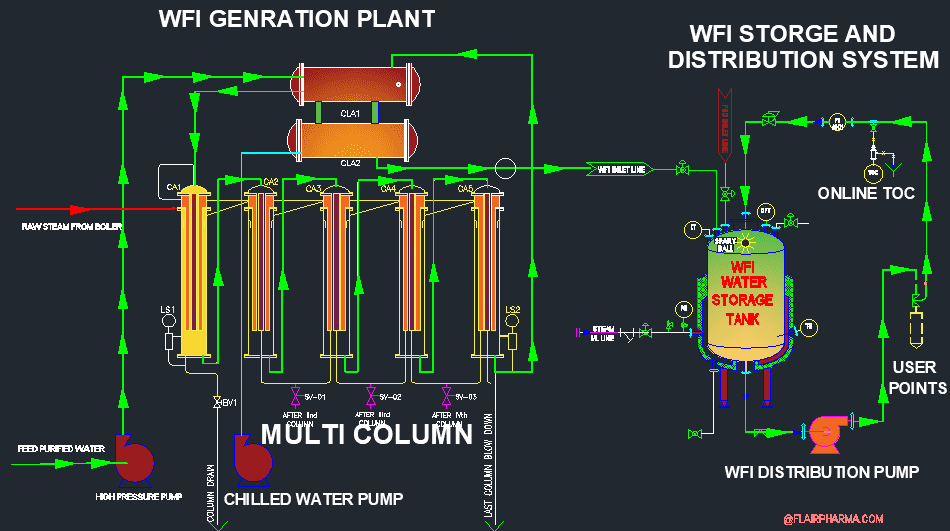
In this system, steam separates from water, leaving dissolved solids, non-volatiles, and high molecular weight impurities behind.
A separator removes fine mist as well as entrained impurities such as endotoxins. Generally, the system used to generate the WFI is called Multi column.
The water operates on the interstage heat exchange principle, which gradually reduces the consumption of heat energy and cooling water.
As a result, it is possible to produce large quantities of Pyrogen-free sterile water for injection. All international standards, like USP, IP, BP, and JP have a description of the WFI and their quality parameters.
WFI quality parameters as per international standards:
Qualities | Parameters |
| Conductivity | <1.3 uS/cm at 25° C |
| Total Organic Carbon | <500 ppb |
| Bacteria | <10 CFU/100ml |
| Endotoxin | <0.25 IU/ml |
Principle of the WFI generation System:
The operating principle of Multi-column is straightforward and efficient in that the Distilling Columns operate at different pressures and temperatures, allowing the energy in the process to be transferred from column to column.
With current technology, Thin Film Evaporation Distillation is the method in which the shell and tube heat exchangers (columns) for heat transfer via falling film evaporation.
Every drop of water produced is heated to extremely high temperatures, ensuring the sterility levels required for WFI. The Pyrogenic load is separated using centrifugal forces generated by the upward movement of the steam.
The feed-purified water within the plant is constantly moving during the process, so there is no stagnancy. The last column is continuously purged of impurities as well as the Pyrogenic load.
The residual accumulated water will also drain off before Water For Injection is produced, during start-up, during pre-heating, and self-sanitizing. Water for injections can be produced using one or more evaporation columns ranging from 2 to 7 in number.
The number of distillation effects is also determined by the number of distillation columns (therefore, the term “multi-effect distillation”).
As the number of columns increases, utility consumption decreases, and thus the unit’s operating costs decrease while investment costs increase.

Breakeven structure for the WFI generation and Distribution System:
- Operation procedures.
- Maintenance procedures.
- Qualification (DQ, FAT, IQ, OQ,)
- Validation (three phase Qualifications).
- Monitoring (Quality Monitoring).
- Control strategy.
- System LifecycleDesign
- Cleaning/Sanitisation
WFI generation and distribution online water quality Monitoring:
- TOC (Online TOC instrument)
- Conductivity (online Conductivity Meter)
- Temperature, Flow & pressure.
- Microbiological purity will check by Grab sample and test my microbiology lab taking a minimum of 5 days.
- Offline Endotoxin testing will do in Lab.
Design requirement and functional specification of WFI plant:
- WFI plant will be made with SS 316L (DIN 1.4435) stainless steel with active surfaces being electro-polished.
- All interconnections are joined with Orbital welding.
- The First Heat exchanger/ Column has a double tube sheet where the boiler’s raw steam will use.
- All Columns are insulated with glass wool covered with the AISI 304 stainless steel sheet.
- All contact parts which are directly in contact with sterile water or WFI are electro-polished with state-of-the-art technology.
- All distribution piping lines are manufactured with SS 316L and electro-polished pipes being orbital welded with sanitary fittings and pharmaceutical-grade gaskets will be used if any.
Qualification of Water for Injection in Pharma:
The qualification of the purified water is held in three phases as per the following details:
- Phase 1 (investigational phase): A test period of 2-4 weeks – monitor the system. System to operate continuously without failure or performance deviation. Chemical and microbiological testing should include in accordance with a defined plan.
- Phase 2 (verification step): A further test period of 2-4 weeks – further intensive monitoring of the system utilization of all the sops after the satisfactory completion of phase 1 sampling scheme generally the same as in phase 1 water can be used for manufacturing purposes during this phase
- Phase 3 (Final Approval): Over 1 year after the satisfactory completion of phase 2, Water for Injection can be used for manufacturing purposes during this phase, Demonstrate: Extended reliable performance That seasonal variations are evaluated. Sample locations, sampling frequencies, and tests should be reduced to the normal routine pattern based on established procedures proven during phases 1 and 2.
Best Manufacturers of Water For Injection Systems in India:
- Praj Hi purity
- TSA
- Hydropure
- CN waters
- SANPURE SYSTEMS PVT LTD
- Indu ionpure
- Omea Scientific Instruments
FAQ:-
Which water is used for injection?
Answer:- Water for Injection (WFI) is used for the addition of an appropriate solute, sterile, nonpyrogenic, distilled water in a single dose bottle is known as sterile water for injection, is already covered in USP, BP, IP, and every pharmacopeia. It can also be utilized as a dispenser for diluent. For all types of injectables like Dry powder, liquid injection, and Vaccines WFI is used as an excipient for all injectables.
What is water for injection made of?
Answer: WFI is made up of pharmacopeial purified water, by converting purified water to pure steam and then again condensing this pure steam to water and this water is called Water for Injection. This WFI made at 125 degrees that’s why it is also called sterile, nonpyrogenic, solute-free preparation of distilled water for injection is known as sterile water for injection.
How to make water for injection?
Answer: Injection water is free of pyrogens. It has no additional content. By distilling purified water in many columns, water for injections can be made.
What are the benefits of water for injection?
Answer: As previously mentioned, WFI is a type of sterile water used to provide pharmaceuticals or medications intravenously to patients. It serves as a cleaning agent in addition to being used to create solutions. It is used to clean anything that will come into contact with the medicine due to its highly pure nature. Any vials, caps, stoppers, ampules, and notably the machinery needed to prepare and store the medications fall under this category.
For more information on water system-related setup, please write us at: admin@flairpharma.com

1 thought on “Best Technology of WFI Generation and Distribution Plant”