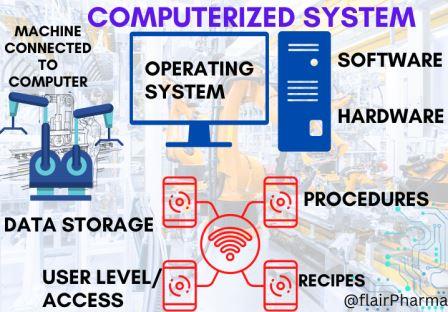
Good Automated Manufacturing Practice (GAMP) in the pharmaceutical realm is a multifaceted and intricate framework of guidelines and principles. It stands as a testament to the industry’s commitment to precision and quality in the era of automation. GAMP is not merely a set of rules but a complex ecosystem of standards devised to ensure that automated systems within pharmaceutical manufacturing maintain the highest levels of reliability, integrity, and compliance.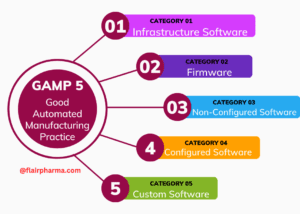
Scope and Objectives of GAMP
The Scope and Objectives of GAMP 5 (Good Automated Manufacturing Practice) represent a pivotal framework within the pharmaceutical and manufacturing industries. GAMP 5, as the fifth iteration of this essential guideline, extends its influence over a wide spectrum of automated systems and processes, with the overarching aim of ensuring product quality, patient safety, and regulatory compliance. Let’s delve into the intricacies of the scope and objectives of GAMP 5.
Scope of GAMP :
- Pharmaceutical and Beyond: GAMP 5’s scope transcends the pharmaceutical industry, encompassing various sectors where automation plays a critical role. While it finds its roots in pharmaceuticals, it has been adapted for use in biotechnology, medical device manufacturing, and other highly regulated industries.
- Lifecycle Approach: GAMP 5 adopts a comprehensive, lifecycle-based approach. It covers the entire lifecycle of automated systems, from concept and design through to retirement, ensuring that quality is maintained at every stage.
- Global Applicability: GAMP 5 is internationally recognized and accepted. It is not limited to a specific region or regulatory body, making it a versatile guideline that can be applied globally.
- Adaptability: GAMP 5’s scope is adaptable, catering to systems of varying complexity and criticality. Whether it’s a simple data logging system or a complex manufacturing control system, GAMP 5 can be tailored to suit the specific needs of the automated system in question.
Objectives of GAMP:
- Risk-Based Approach: GAMP 5 adopts a risk-based approach to validation, focusing resources on areas of highest risk. The objective is to identify and mitigate potential risks associated with automated systems to ensure product quality and patient safety.
- Consistency and Reproducibility: GAMP 5 seeks to establish consistency and reproducibility in automated processes. This ensures that manufacturing processes are not only reliable but also consistently deliver products that meet quality standards.
- Compliance: A fundamental objective of GAMP 5 is regulatory compliance. It aims to help organizations adhere to the relevant regulations and guidelines, such as those set forth by regulatory authorities like the FDA (Food and Drug Administration) and the EMA (European Medicines Agency).
- Documentation and Traceability: GAMP 5 emphasizes the importance of thorough documentation and traceability throughout the system lifecycle. The objective is to maintain a clear record of system specifications, design, testing, and maintenance to facilitate audits and inspections.
- Continuous Improvement: GAMP 5 encourages a culture of continuous improvement. It promotes the use of best practices and the incorporation of new technologies to enhance the efficiency and effectiveness of automated systems.
- Efficiency: While ensuring compliance and quality, GAMP 5 also aims to optimize the use of resources. It seeks to strike a balance between regulatory requirements and the efficient operation of automated systems.
In summary, the scope and objectives of GAMP 5 underscore its significance in highly regulated industries. It provides a versatile framework that covers a broad spectrum of automated systems and processes, with the ultimate goal of ensuring product quality, patient safety, and compliance with global regulatory standards. Through a risk-based approach, consistency, and continuous improvement, GAMP 5 stands as a guiding beacon for organizations navigating the complex landscape of automated manufacturing.
History of the Good Automated Manufacturing Practice GAMP
- 1980s: The concept of GAMP begins to take shape in the pharmaceutical industry as computerized systems become more prevalent in manufacturing and quality control processes.
- 1991: The first official GAMP guidance document, known as “GAMP 1,” is published by the International Society for Pharmaceutical Engineering (ISPE). It primarily focuses on the validation of automated systems.
- 1995: GAMP 2 is released, providing more comprehensive guidance on computerized systems validation. This version expands its scope to include the principles of risk management.
- 2000: GAMP 3 is introduced, further emphasizing risk-based approaches to validation and incorporating best practices from various industries.
- 2003: GAMP 4 is published, placing a greater emphasis on the use of automated systems in the context of good manufacturing practices (GMP) and regulatory compliance.
- 2008: GAMP 5 is released, marking a significant evolution in the framework. It introduces a more structured approach to risk management and adopts a lifecycle perspective for validation.
- 2017: GAMP 5 continues to be a cornerstone of computerized systems validation in pharmaceutical and related industries, guiding organizations in the implementation of risk-based approaches and compliance with evolving regulations.
- Ongoing: The GAMP Community of Practice, comprising professionals from various industries, continues to develop and update GAMP guidance documents to align with technological advancements and changing regulatory landscapes.
The GAMP framework has evolved over the years to address the growing complexity of automated systems, emphasizing risk management, compliance, and the need for a lifecycle approach to validation. It remains an essential resource for organizations seeking to ensure the quality, safety, and regulatory compliance of their computerized systems.
Key Principles of GAMP:
GAMP 5 (Good Automated Manufacturing Practice) is founded on a set of key principles that serve as the cornerstone of its guidelines for the pharmaceutical and related industries. These principles are designed to ensure the reliable operation of automated systems and the consistent production of high-quality products while adhering to regulatory requirements. Let’s explore the key principles of GAMP 5:
- Lifecycle Approach: GAMP 5 adopts a lifecycle approach, emphasizing that the validation process should begin during the early stages of system development and continue throughout its entire lifecycle. This principle ensures that the system remains in a validated state, even as it undergoes changes and updates.
- Risk-Based Approach: Central to GAMP 5 is the concept of a risk-based approach to validation. It recognizes that not all aspects of a system or process are equally critical. Instead, resources and efforts should be focused on areas with the highest potential risk to product quality and patient safety.
- Validation Planning: GAMP 5 promotes the development of a comprehensive validation plan that outlines the scope, objectives, and methodologies for validation activities. This plan acts as a roadmap for validation efforts and ensures consistency and clarity throughout the validation process.
- User and Supplier Collaboration: Collaboration between system users and suppliers is encouraged. This principle emphasizes the importance of clear communication and cooperation between those who operate the automated system and those who provide and maintain it.
- Verification and Documentation: Verification is a key component of GAMP 5, involving the systematic testing and documentation of the automated system’s functions. Comprehensive documentation is crucial to provide evidence of system compliance and validation.
- Change Control and Management: GAMP 5 recognizes that changes are inevitable in the lifecycle of an automated system. It promotes effective change control processes to ensure that any modifications or updates do not compromise the system’s validated state.
- Data Integrity: Ensuring the integrity of data generated by automated systems is a critical principle. GAMP 5 places a strong emphasis on data security, accuracy, and reliability, particularly in pharmaceutical processes where data integrity is paramount.
- Compliance with Regulations: Compliance with regulatory requirements is a fundamental principle of GAMP 5. It acknowledges the need for organizations to adhere to regional and global regulations, such as those outlined by the FDA (Food and Drug Administration) and other regulatory bodies.
- Training and Competency: GAMP 5 underscores the importance of training and competency of personnel involved in system operation and validation. Well-trained individuals are essential to maintaining the integrity of automated processes.
- Documentation and Records Management: Proper documentation and records management are vital to demonstrate compliance and traceability. GAMP 5 mandates the creation and maintenance of clear and organized records throughout the system’s lifecycle.
- Quality Risk Management: Quality risk management principles are integrated into GAMP 5, helping organizations identify, assess, and mitigate risks associated with automated systems. This proactive approach enhances product quality and patient safety.
- Continuous Improvement: Lastly, GAMP 5 encourages organizations to adopt a culture of continuous improvement. It promotes the ongoing evaluation of automated systems and validation processes to identify opportunities for enhancement and optimization.
These key principles form the foundation of GAMP 5, providing a comprehensive and adaptable framework for organizations seeking to implement and validate automated systems while maintaining the highest standards of quality, safety, and compliance.
List of the GAMP guidance:
The GAMP (Good Automated Manufacturing Practice) guidance series consists of several documents that provide detailed guidance on different aspects of computerized systems validation in regulated industries, primarily pharmaceutical and healthcare. As of my last knowledge update in September 2021, here is a list of some key GAMP guidance documents:
- GAMP 5: A Risk-Based Approach to Compliant GxP Computerized Systems: This is the primary and most comprehensive GAMP guidance document. It outlines the principles of a risk-based approach to computerized systems validation.
- GAMP Good Practice Guide: Validation of Laboratory Computerized Systems (Second Edition): Focuses on the validation of computerized laboratory systems, including analytical instruments and data management systems.
- GAMP Good Practice Guide: IT Infrastructure Control and Compliance: Provides guidance on how to control and validate IT infrastructure components that support computerized systems.
- GAMP Good Practice Guide: Electronic Data Archiving: Addresses the validation and maintenance of electronic data archiving systems, which are critical for data integrity and compliance.
- GAMP Good Practice Guide: Manufacturing Execution Systems: Offers guidance on the validation and use of Manufacturing Execution Systems (MES) in pharmaceutical manufacturing.
- GAMP Good Practice Guide: Validation of Process Control Systems: Focuses on the validation of process control systems used in manufacturing and related processes.
- GAMP Good Practice Guide: IT Infrastructure Management and Control: Provides recommendations for managing and controlling IT infrastructure in a GxP environment.
- GAMP Good Practice Guide: Working with Suppliers: Offers guidance on collaborating effectively with suppliers and managing supplier relationships within the context of computerized systems validation.
- GAMP Good Practice Guide: Validation of Analytical Instrument Qualification (AIQ) Systems: Focuses on the validation of analytical instrument qualification systems used in laboratory settings.
- GAMP Good Practice Guide: Validation and Compliance of Computerized GCP Systems: Addresses the validation and compliance of computerized systems used in Good Clinical Practice (GCP) environments.
Please note that the GAMP guidance documents may evolve over time, with new editions or updates being released. Therefore, it’s essential to check the latest resources provided by ISPE (International Society for Pharmaceutical Engineering) or the GAMP Community of Practice for the most up-to-date information and guidance on computerized systems validation.
GAMP 5 Categories:
| GAMP 5 Category | Description |
|---|---|
| Category 1: Infrastructure Software | Software used for basic infrastructure services, such as operating systems, network protocols, and database management systems. |
| Category 2: Non-configured Software | Commercial off-the-shelf (COTS) software that is not configured or customized for specific use but may require qualification or testing. |
| Category 3: Configured Software | COTS software that is configured or customized for specific use. Qualification and validation are typically required to ensure proper functionality. |
| Category 4: Bespoke Software | Custom-developed software designed for specific purposes. Comprehensive validation is essential due to its unique nature. |
| Category 5: Non-embedded Software | Software not embedded within a hardware device, including standalone software applications or systems. |
| Category 6: Embedded Software | Software that is an integral part of a hardware device, often requiring validation in conjunction with the entire device. |
| Category 7: Firmware Software | Software embedded in hardware devices, often requiring validation as part of the device’s overall functionality and safety. |
| Category 8: Non-product Software | Software that does not directly impact product quality, safety, or efficacy but is still subject to validation and control to ensure data integrity and compliance. |
| Category 9: Product Software | Software directly linked to product quality, safety, or efficacy, and critical for compliance with regulatory requirements. Extensive validation is imperative. |
| Category 10: Infrastructure Hardware | Physical hardware components that form part of the infrastructure, such as servers, network equipment, and data storage devices. |
| Category 11: Non-configured Hardware | Hardware devices, such as laboratory instruments, that are not customized or configured for specific use but require qualification or testing. |
| Category 12: Configured Hardware | Hardware devices customized or configured for specific purposes, necessitating qualification to ensure proper performance. |
| Category 13: Bespoke Hardware | Custom-designed hardware devices or systems, requiring comprehensive qualification due to their unique nature. |
Benefits of GAMP
| Benefits of GAMP 5 | Description |
|---|---|
| 1. Enhanced Product Quality | GAMP 5 helps ensure consistent product quality by rigorously validating automated systems. |
| 2. Improved Patient Safety | Compliance with GAMP 5 principles reduces the risk of errors in pharmaceutical and medical device manufacturing. |
| 3. Regulatory Compliance | Adhering to GAMP 5 facilitates compliance with regulatory standards like FDA and EMA guidelines. |
| 4. Risk-Based Approach | GAMP 5’s risk-based approach focuses resources on areas of highest risk, optimizing validation efforts. |
| 5. Lifecycle Perspective | It covers the entire system lifecycle, from development to retirement, ensuring ongoing validation. |
| 6. Effective Change Control | GAMP 5 principles help manage changes and updates to systems without compromising their validated state. |
| 7. Clear Documentation | Comprehensive documentation ensures transparency, traceability, and ease of audit and inspection. |
| 8. Data Integrity Assurance | Robust data management practices prevent data corruption and maintain data integrity. |
| 9. Supplier Collaboration | Collaboration with suppliers ensures access to necessary documentation and support for validation. |
| 10. Efficient Resource Utilization | GAMP 5 balances compliance and efficiency, optimizing resource allocation for validation activities. |
| 11. Continuous Improvement Culture | Encourages a culture of continuous improvement, leading to enhanced system efficiency and effectiveness. |
| 12. Versatility Across Industries | Applicable beyond pharmaceuticals, GAMP 5 can be adapted for use in various automated industries. |
Implementation Challenges of GAMP 5
The implementation of GAMP 5 (Good Automated Manufacturing Practice) presents organizations with several notable challenges. While GAMP 5 offers a comprehensive framework for the validation and management of automated systems, its successful adoption requires careful planning and execution. Here are some key implementation challenges that organizations may encounter:
- Resource Allocation: Implementing GAMP 5 often requires a significant allocation of resources, including personnel, time, and financial investments. Organizations may face challenges in securing the necessary resources, especially in terms of skilled personnel and adequate budgets for validation activities.
- Complexity of Automation: Modern automated systems can be highly complex, and validating their functionality and compliance with GAMP 5 principles can be a daunting task. Organizations may struggle to fully understand the intricacies of these systems and how they fit within the GAMP framework.
- Documentation Burden: GAMP 5 places a strong emphasis on documentation, which can be burdensome for organizations. Creating and maintaining comprehensive validation documentation requires attention to detail and a commitment to thorough record-keeping, which can be time-consuming.
- Change Management: Organizations often encounter challenges when implementing changes or updates to automated systems while maintaining their validated state. GAMP 5’s principles of change control and management require careful planning and execution to avoid disruptions to production.
- Training and Competency: Ensuring that personnel are adequately trained and competent in GAMP 5 practices can be challenging. Training programs may need to be developed and implemented, and organizations must continuously assess and improve the skills of their staff.
- Data Integrity: Maintaining data integrity, a core principle of GAMP 5, can be a significant challenge. Organizations must implement robust data management systems and practices to prevent data corruption or manipulation, which can have serious consequences in regulated industries.
- Regulatory Compliance: Achieving and maintaining compliance with regulatory requirements is a constant challenge. Regulatory agencies like the FDA and EMA regularly update their guidelines, and organizations must stay informed and adapt their GAMP 5 practices accordingly.
- Supplier Relationships: Collaborating effectively with system suppliers and vendors, another key principle of GAMP 5, can be challenging. Clear communication and cooperation are essential to ensure that suppliers provide necessary documentation and support for validation efforts.
- Resource Constraints: Some organizations may face resource constraints, limiting their ability to implement GAMP 5 comprehensively. This can lead to potential gaps in validation practices and compromise the overall effectiveness of the system.
- Balancing Efficiency and Compliance: Striking a balance between efficiency and compliance can be difficult. Organizations may be tempted to cut corners to expedite production, but this can risk compromising product quality and patient safety.
- Continuous Improvement: The principle of continuous improvement, while valuable, can be challenging to implement systematically. Organizations must establish mechanisms for ongoing evaluation and enhancement of automated systems and validation processes.
In conclusion, while GAMP 5 offers a robust framework for the validation and management of automated systems, organizations may encounter various implementation challenges. Addressing these challenges requires a commitment to resource allocation, training, documentation, and a proactive approach to risk management. Successfully navigating these obstacles is crucial for organizations operating in regulated industries to ensure the reliability, quality, and compliance of their automated systems.
GAMP 4 vs GAMP 5
| Aspect | GAMP 4 | GAMP 5 |
|---|---|---|
| Publication Year | 2001 | 2008 |
| Risk-Based Validation | Introduced the concept of risk-based validation. | Enhanced the application of risk-based validation, making it a central theme. |
| Flexibility | Relatively prescriptive and rigid, sometimes challenging to adapt. | More flexible and pragmatic, providing adaptability to different system types and industry needs. |
| Documentation Focus | Strong emphasis on documentation, sometimes overshadowing quality considerations. | Shifted focus from documentation-centric to a holistic approach, considering overall product and process quality. |
| Quality Risk Management | Introduced risk-based validation but with less emphasis on quality risk management. | Strong emphasis on quality risk management, systematically assessing and mitigating risks. |
| Supplier Management | Limited focus on supplier management. | Recognizes the significance of supplier management and encourages effective collaboration. |
| Continuous Improvement | Limited emphasis on continuous improvement. | Promotes a culture of continuous improvement, reflecting the importance of ongoing system optimization. |
| Alignment with Standards | Largely independent of international standards. | Aligns more closely with international standards and guidelines, ensuring harmonization with global best practices. |
| Process Understanding | Less focus on the importance of product and process understanding. | Emphasizes the significance of understanding the product and process. |
| Adaptability | Less adaptable due to its prescriptive nature. | More adaptable, making it easier to apply to diverse system types and industry contexts. |
Frequently Asked Questions:
What is GAMP, and what does it stand for?
Answer: GAMP stands for Good Automated Manufacturing Practice. It is a set of guidelines and best practices for computerized systems validation in regulated industries.
What is the primary objective of GAMP in a technical context?
Answer: GAMP’s primary technical objective is to ensure the reliability and compliance of computerized systems used in highly regulated environments, such as pharmaceutical manufacturing.
How does GAMP address risk management in technical terms?
Answer: GAMP incorporates a risk-based approach to validation, where technical assessments identify and prioritize areas of highest risk, allowing for targeted validation efforts.
What are the key technical categories in GAMP 5 for classifying software and hardware components?
Answer: GAMP 5 introduces 13 technical categories, including Infrastructure Software, Configured Software, Bespoke Software, Embedded Software, and others, to classify software and hardware components used in automated systems.
What is Quality Risk Management (QRM) in GAMP, and why is it important from a technical standpoint?
Answer: QRM is a technical approach that systematically identifies, assesses, and mitigates risks throughout the validation process. It’s crucial from a technical perspective to ensure the reliability and safety of automated systems.
How does GAMP 5 promote technical collaboration with suppliers?
Answer: GAMP 5 encourages technical collaboration with suppliers by emphasizing clear communication, documentation, and sharing of technical information, ensuring that supplier components align with validation requirements.
What technical documentation is typically required in a GAMP-compliant validation process?
Answer: Technical documentation may include User Requirement Specifications (URS), Functional Specifications (FS), Design Specifications (DS), Test Protocols, and technical reports that demonstrate compliance with validation requirements.
What role does technical change control play in GAMP, and why is it significant?
Answer: Technical change control in GAMP manages modifications or updates to automated systems while ensuring they remain in a validated state. It’s critical to maintain technical compliance and data integrity.
How does GAMP 5 address technical alignment with international standards and guidelines?
Answer: GAMP 5 emphasizes technical alignment with international standards and guidelines, such as ISO 9000, ICH Q8, Q9, Q10, and FDA 21 CFR Part 11, to ensure harmonization of technical practices.
What technical practices are encouraged for ensuring data integrity in GAMP-compliant systems?
Answer: Technical practices for data integrity include audit trails, electronic signatures, access controls, and secure data storage, all of which are crucial for maintaining data integrity in automated systems.
How does GAMP 5 promote a culture of technical continuous improvement?
Answer: GAMP 5 encourages a technical culture of continuous improvement by promoting the evaluation of automated systems, validation processes, and the incorporation of new technologies to enhance efficiency and effectiveness.
Can GAMP be applied to industries other than pharmaceuticals from a technical standpoint?
Answer: Yes, GAMP’s technical principles can be adapted for use in various industries, including biotechnology, medical devices, food manufacturing, and more, wherever computerized systems require validation in a regulated environment.