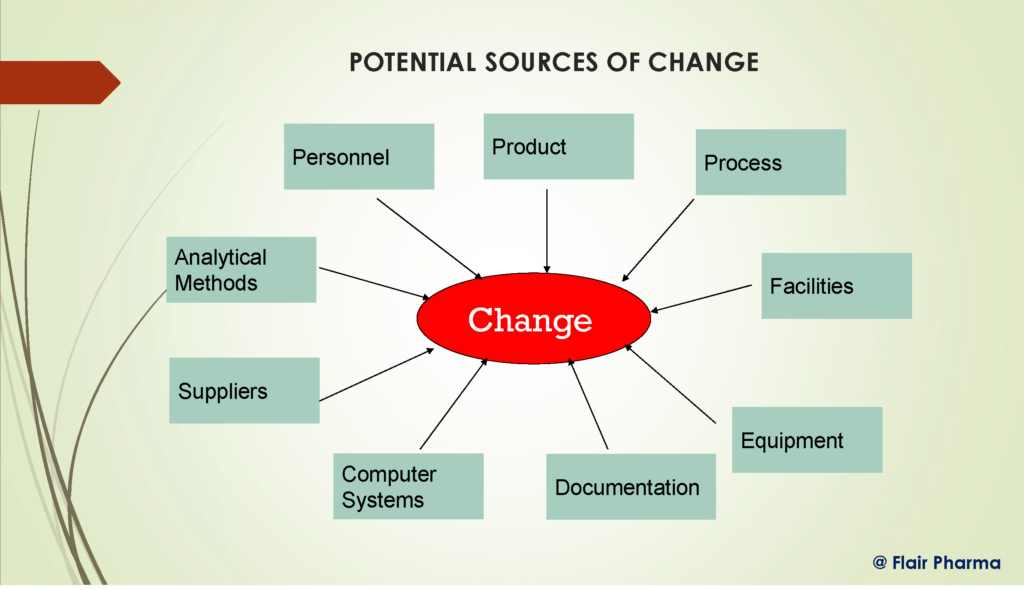
Change Control in pharma industries is the main Quality Management System tool. Every change in the equipments, raw materials, facilities, utilities, designs, formulas, procedures, packaging, labeling, computer systems, and any related paperwork is referred to as a change ( SOPs, quality manual, etc.) “Change control is a formal method by which qualified representatives of appropriate disciplines examine proposed or actual changes that may have an impact on a validated status,” according to the WHO. The goal is to ascertain whether a particular activity is required to keep the system in a validated state.
“CHANGE”
- usually refers to a planned alteration.
- documented.
- considered before implementation.
- Normally, changes are either permanent or have a fixed period of validity.
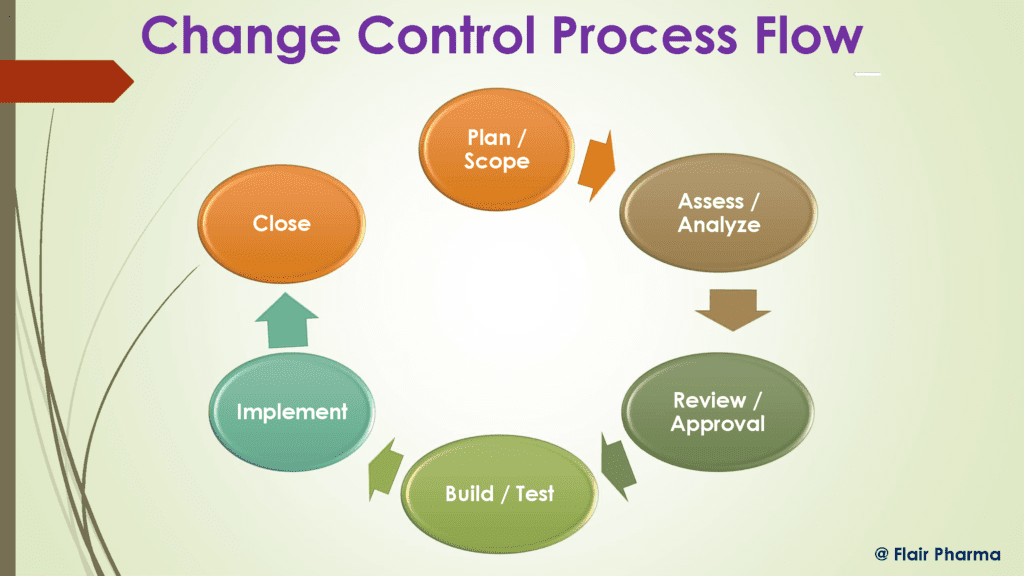
Working Principle of the Change Control
It seems sensible that quality assurance would be in charge of change control since it is regarded as a crucial component of the pharmaceutical quality assurance system (Q.A representative, Q.A head).
Change management is a company-wide responsibility rather than a department-specific one.
The change control department keeps track of any changes that might have an impact on the process or product quality and either determines not to execute a change or specifies the steps required to do so.
Purpose of Change Control System
- Prevention of non-authorized changes.
- Evaluation of proposed changes.
- Assessment of necessary actions (including qualifications).
- Notification to all interested parties in the company.
- Notification to regulatory authorities as necessary.
- Documentation of change and decision-making process Controlling the Changes.
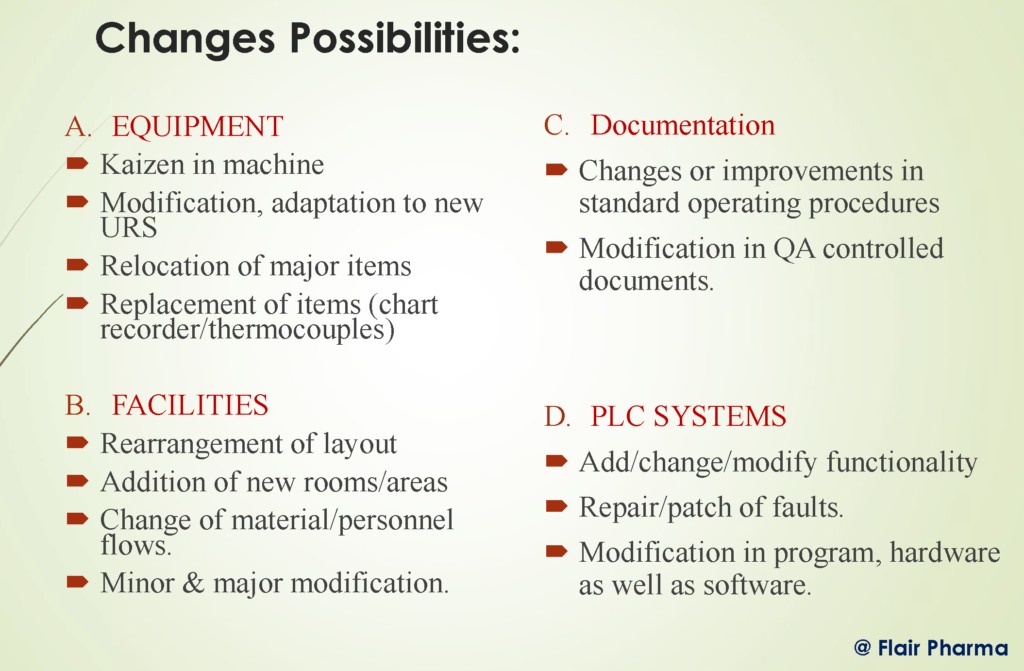
WHEN IS A CHANGE “CRITICAL”?
- The component is in direct contact with the product (e.g. valves).
- Components can affect product quality (e.g. stirrers, manufacturing vassals).
- Records important process data (e.g. temperature/pressure).
- Provides alarms.
- Can influence the quality and performance of support systems (e.g. water, steam, HVAC).
- Provides access control to protected systems (CSV).
- Provides process control (e.g. recipes, controllers).
- Proves status control (e.g. SAP, BPICS).
KEY FEATURES OF A CHANGE CONTROL SYSTEM
- CHANGE REQUEST/NOTIFICATION
- Standardized form(s)/system.
- A clear understanding of what is a change.
- Local decision-making competence.
- CHANGE ASSESSMENT
- Change committee.
- All relevant persons are involved.
- Clear decision-making process resulting in actions.
- Cost-to-benefit considerations are essential!
- CHANGE IMPLEMENTATION
- Clear allocation of responsibilities, timelines.
- (milestones for complex changes).
- Overall QA coordination.
- Full documentation of actions.
- FOLLOW-UP
- Regular follow-up to ensure all parties have acted in accordance with decisions Standardized form(s).
- Review documentation.
- TRACEABILITY
- Ensure an internal database providing.
- Easy review and tracking of change
Categorization of the Changes in the Change Control in pharma
There are mainly 3 types of Change Categories in pharma organizations, They are categorized according to their impact on the resulting product, process, or system. as following:
- Minor
- Major
- Critical
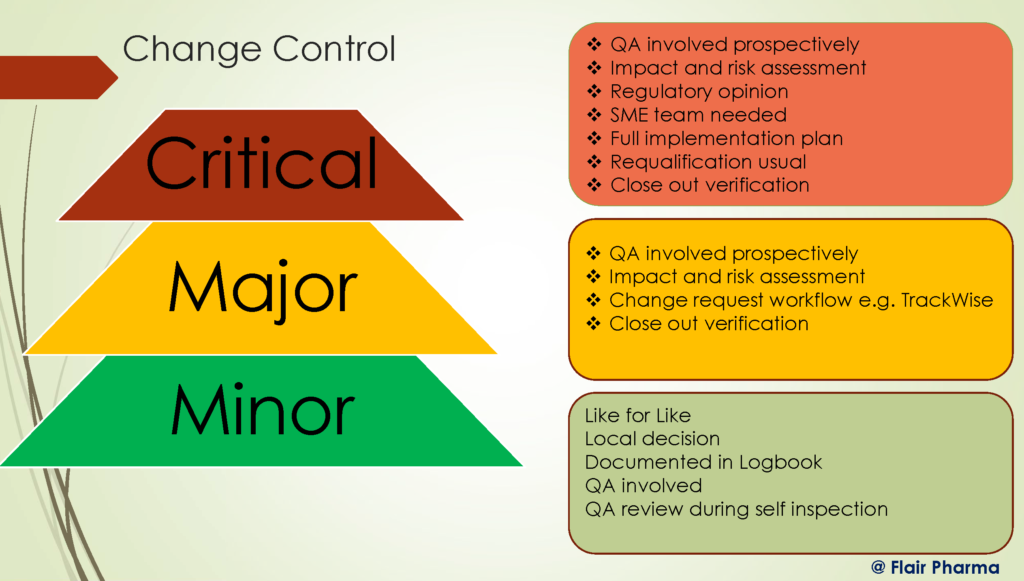
- Minor Change
The features of the final product are unaffected by a modest alteration that affects just a small portion of the process. All of the quality standards that were in place before the change’s implementation are still there in the finished product. For instance, changing a mixing machine in the production department to a faster machine has little effect on the attributes of the finished product. The only parameter that might change is manufacturing time, with all other factors remaining unchanged. - Major Change
A significant modification affects a product, facility, process, system, or piece of equipment’s Current Good Manufacturing Practice (cGMP) characteristics. The reliability of the process may be impacted by these changes, but they can be mitigated with the right precautions or testing techniques. For instance, it would be considered a big modification if the Quality Department decided to raise the sterilization temperature for a specific product from 100⁰C to 121⁰C. - Critical Change
A significant alteration may negatively affect the product’s quality and prevent it from providing the end customer with the desired features. It is impossible to test this change further or make improvements because it cannot be undone.
For instance, it is crucial if a change in the product’s formulation results in an unfavorable change in the color of the product. The impacted item needs to be removed from the market because it cannot be utilized for human consumption.
Change Control – Traceability
- WHO made the change.
- WHY was change necessary or desirable?
- WHEN was the change implemented?
- WHAT internal consequences arise:
- Risk/benefit assessments.
- Cost/benefit assessments.
- Regulatory impact.
- Who gave APPROVALS
Full documentation of the change process to allow clarity in decision-making, information flow, and responsibilities.
Problems with change control systems
- No formal close-out procedure to ensure all actions are completed by the QA unit.
- Changes implemented before all actions closed.
- Equipment changes resulting from breakdown and maintenance are not systematically captured or reviewed by Quality Unit.
- Poor documentation and traceability of changes
- Too many changes open at the same time.
A famous book by Mr. The International Bestseller: DO IT TO DAY Are you also tired of putting off your dreams until “tomorrow?” Guess what! Tomorrow never comes Do It Today: Overcome procrastination, improve productivity and achieve more meaningful things
Frequently Asked Questions

6 thoughts on “Change Control in Pharma 2023”